Liquid chromatographic analysis method for parecoxib-sodium related substances
A liquid chromatographic analysis, parecoxib sodium technology, applied in analytical materials, material separation, measuring devices and other directions, can solve the problems of relying on instruments, unable to accurately and effectively quantitatively detect impurities, and unable to guarantee the quality of drugs, and achieve the goal of reducing The effect of testing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
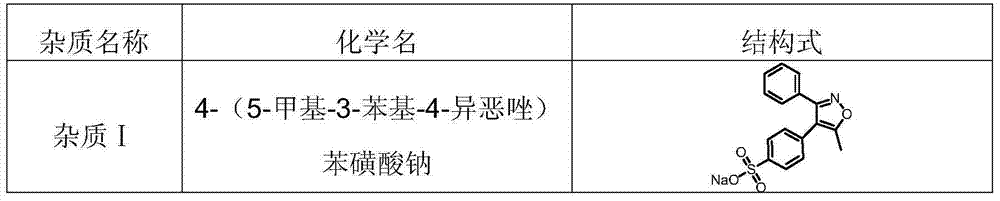
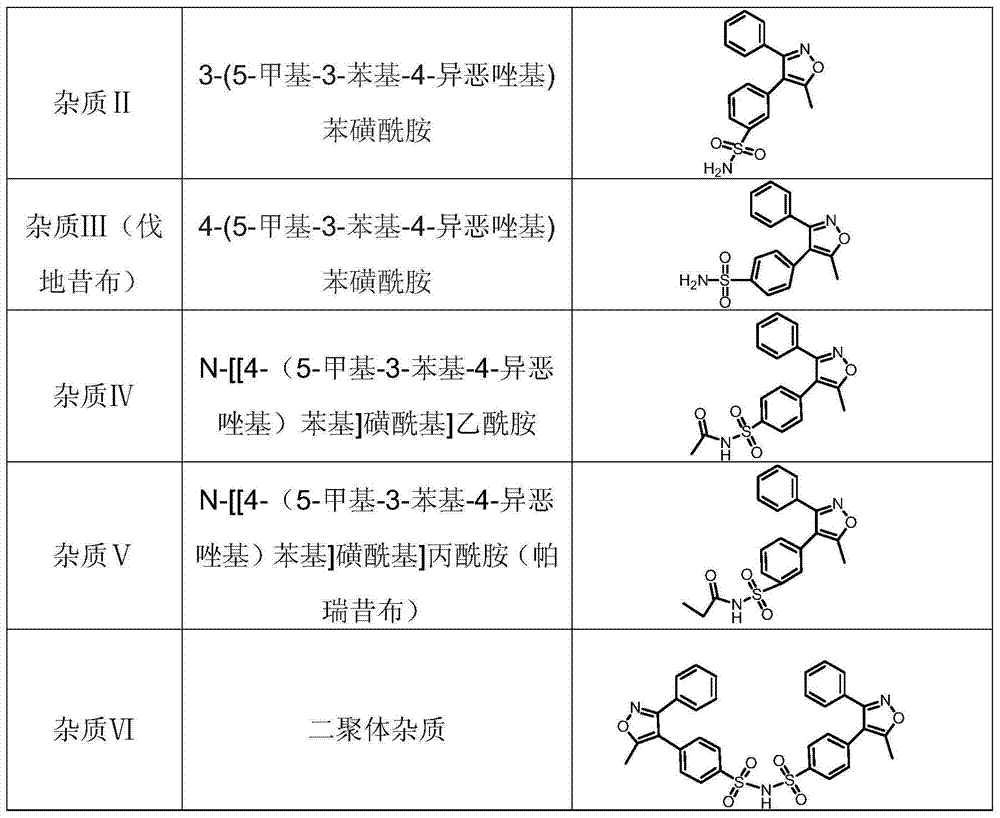
[0033] Step 1: System suitability test: (1) Take the reference substances of impurity Ⅰ, impurity Ⅱ, impurity Ⅲ, impurity Ⅳ, impurity Ⅴ, impurity Ⅵ and parecoxib sodium respectively, and acetonitrile aqueous solution (volume ratio 40:60) Dilute to a mixed solution containing 1 mg of parecoxib sodium, 1.5 μg of impurity I, 1.5 μg of impurity II, 1.5 μg of impurity III, 1.5 μg of impurity IV, 1.5 μg of impurity V, and 1.5 μg of impurity VI per ml, as a system suitability solution (2) Set the liquid chromatograph, wherein the chromatographic column is a 100mm × 4.6mm silica gel column, and the built-in C18 core-shell technology is bonded with pentafluorophenyl filler, and the particle size of the filler is 2.6 μm, and the liquid chromatograph The detection wavelength of the instrument is 230nm, water-methanol-trifluoroacetic acid (volume ratio is preferably 10:90:0.05) as mobile phase A, water-methanol-trifluoroacetic acid (volume ratio is preferably 90:10:0.05) It is mobile phas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com