Preparation method of parecoxib sodium compound as well as intermediate impurity and application of parecoxib sodium compound
A technology of parecoxib sodium and compound is applied in the field of preparation of parecoxib sodium compound, which can solve the problem of low synthesis yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
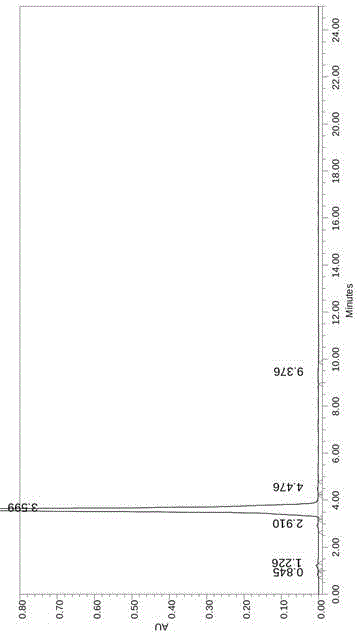
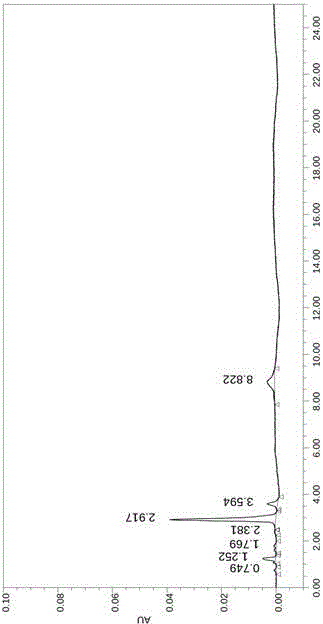
Image
Examples
Embodiment 13
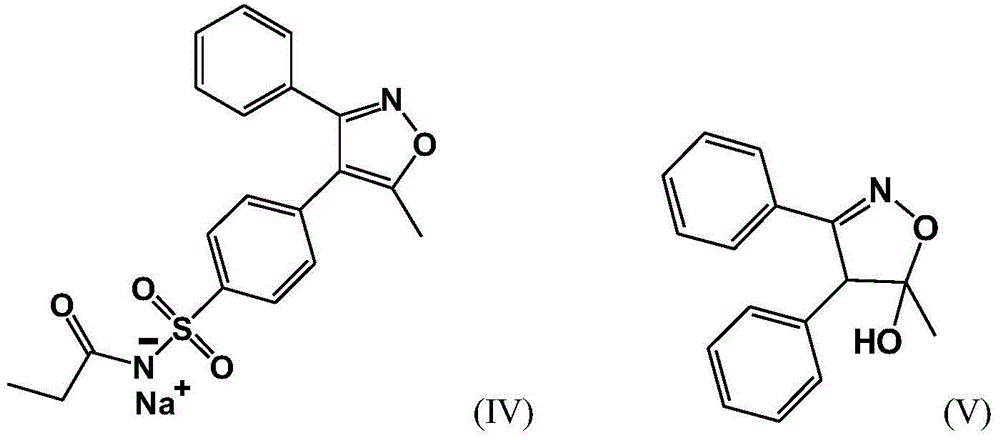
[0046] The preparation of embodiment 13-methyl-4,5-diphenyl-4,5-dihydroisoxazol-5-alcohol
[0047] (1) Add 71.3g of 1,2-benzophenone, 64.6g of tetrahydropyrrole, 300mL of cyclohexane and 0.2g of glacial acetic acid into a 1L three-necked flask, heat up to reflux, and water will come out from the water separator until Anhydrous was formed to stop the reaction, and the reaction solution was concentrated under reduced pressure at 50°C until no distillate was obtained to obtain about 90 g of the compound of formula I as a yellow oil.
[0048] (2) Put the obtained oil into a 1L reaction flask, add 500mL of acetonitrile and 58g of 2,6-lutidine, dropwise add 112g of acetyl chloride below 15°C, continue the reaction until the raw materials disappear, and obtain the acylated product formula II compound solution.
[0049] (3) Slowly add the solution containing the compound of formula II dropwise to the mixed aqueous solution of hydroxylamine hydrochloride and sodium acetate (101g of hy...
Embodiment 23
[0062] The preparation of embodiment 23-methyl-4,5-diphenyl-4,5-dihydroisoxazol-5-alcohol
[0063] (1) Add 72..5g of 1,2-benzophenone, 75g of tetrahydropyrrole, 350mL of cyclohexane and 0.25g of glacial acetic acid into a 1L three-necked flask, heat up to reflux, and water will come out from the water separator. The reaction was stopped until anhydrous was formed, and the reaction solution was concentrated under reduced pressure at 50°C until no distillate was obtained to obtain about 93.5 g of the compound of formula I as a yellow oil.
[0064] (2) Put the obtained oil into a 1L reaction flask, add 525mL of acetonitrile and 55g of 2,6-lutidine, dropwise add 128g of acetyl chloride below 15°C, continue the reaction until the raw materials disappear, and obtain the acylated product formula II compound solution.
[0065] (3) Slowly add the solution containing the compound of formula II dropwise to the mixed aqueous solution of hydroxylamine hydrochloride and sodium acetate (103...
Embodiment 33
[0068] Preparation of Example 33-Methyl-4,5-diphenyl-4,5-dihydroisoxazol-5-ol
[0069] (1) Add 63.5g of 1,2-benzophenone, 70g of tetrahydropyrrole, 300mL of cyclohexane and 0.18g of glacial acetic acid into a 1L three-necked bottle, heat up to reflux, and water will come out from the water separator until there is no more Water was formed to stop the reaction, and the reaction solution was concentrated under reduced pressure at 50°C until no distillate was obtained to obtain about 82.4 g of the compound of formula I as a yellow oil.
[0070] (2) Put the obtained oil into a 1L reaction flask, add 485mL of acetonitrile and 48g of 2,6-lutidine, dropwise add 118g of acetyl chloride below 15°C, continue the reaction until the raw materials disappear, and obtain the acylated product formula II compound solution.
[0071] (3) Slowly add the solution containing the compound of formula II dropwise to the mixed aqueous solution of hydroxylamine hydrochloride and sodium acetate (98g of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com