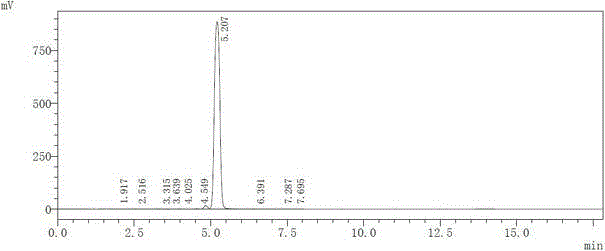
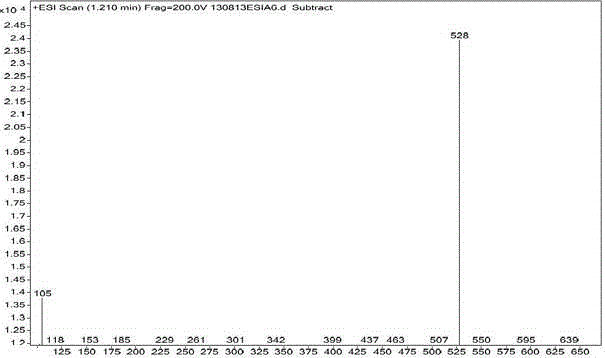
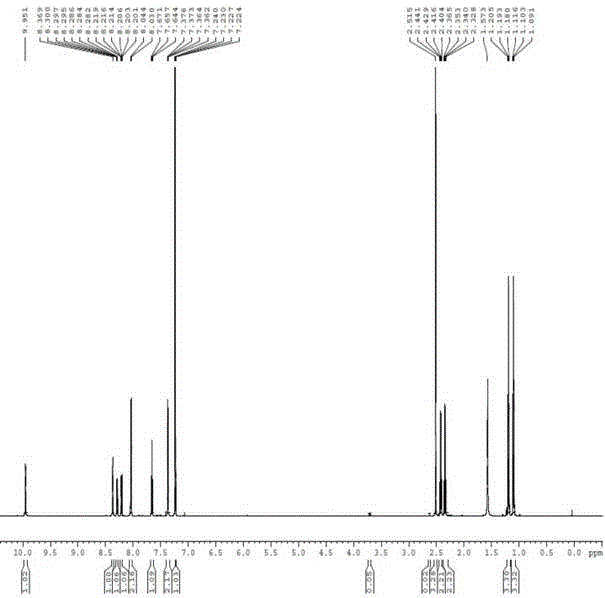
Synthesis method for parecoxib sodium impurity
A technology of parecoxib sodium and a synthesis method, which is applied in the field of chemical pharmacy to achieve the effects of cheap raw materials, improved quality, and improved accurate positioning and characterization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: A parecoxib sodium impurity J(4-N-propionyl[5-methyl-3-(3-N-propionylphenylsulfonamide)-1,2-oxazole-4- base] benzenesulfonamide) synthetic method
[0024] Add 5g of 5-methyl-3,4-diphenylisoxazole, 20g of dichloromethane, and 70g of chlorosulfonic acid into the reaction flask, reflux for 16 hours, add the reaction solution dropwise to 70g of water, 20g of dichloromethane Extract, concentrate to dryness, add 20g ethyl acetate to crystallize for 1.5 hours, filter to get 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonyl chloride 6.88g, yield 74.9%.
[0025] Add 5 g of the above solid and 20 g of dichloromethane into the reaction flask. After the solid dissolves, add dropwise to 20 g of ammonia water, react for 4 hours, concentrate to dryness under reduced pressure, add 15 g of ethanol to reflux for beating, cool to room temperature, and filter to obtain 4-[ 3.76 g of 5-methyl-3-(3-phenylsulfonamide)-1,2-oxazol-4-yl]benzenesulfonamide, yi...
Embodiment 2
[0030] Example 2: A parecoxib sodium impurity J(4-N-propionyl[5-methyl-3-(3-N-propionylphenylsulfonamide)-1,2-oxazole-4- base] benzenesulfonamide) synthetic method
[0031] Add 5g of 5-methyl-3,4-diphenylisoxazole, 20g of dichloromethane, and 100g of chlorosulfonic acid into the reaction flask, reflux for 18 hours, add the reaction solution dropwise to 80g of water, 20g of dichloromethane Extract, concentrate to dryness, add 12g ethyl acetate to crystallize for 2 hours, filter to get 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonyl chloride 7.12g, yield 77.5%.
[0032] Add 5 g of the above solid and 20 g of dichloromethane into the reaction flask. After the solid dissolves, add it dropwise to 15 g of ammonia water, react for 3 hours, concentrate to dryness under reduced pressure, add 15 g of ethanol to reflux for beating, cool to room temperature, and filter to obtain 4-[ 3.60 g of 5-methyl-3-(3-phenylsulfonamide)-1,2-oxazol-4-yl]benzenesulfonamide, ...
Embodiment 3
[0034] Example 3: A parecoxib sodium impurity J(4-N-propionyl[5-methyl-3-(3-N-propionylphenylsulfonamide)-1,2-oxazole-4- base] benzenesulfonamide) synthetic method
[0035] Add 3g of 5-methyl-3,4-diphenylisoxazole, 12g of dichloromethane, and 48g of chlorosulfonic acid into the reaction flask, reflux for 14 hours, and add the reaction solution dropwise to 50g of water, 12g of dichloromethane Extract, concentrate to dryness, add 12g ethyl acetate to crystallize for 2 hours, filter to get 4-[5-methyl-3-(3-phenylsulfonyl chloride)-1,2-oxazol-4-yl]benzenesulfonyl chloride 4.10 g, yield 74.4%.
[0036] Add 4g of the above solid and 16g of dichloromethane into the reaction flask. After the solid is dissolved, add it dropwise to 20g of ammonia water, react for 5 hours, concentrate to dryness under reduced pressure, add 12g of ethanol to reflux for beating, cool to room temperature, and filter to obtain 4-[ 3.18 g of 5-methyl-3-(3-phenylsulfonamide)-1,2-oxazol-4-yl]benzenesulfonamid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com