Synthesis method for parecoxib sodium impurity
A technology of parecoxib sodium and its synthesis method, which is applied in the field of chemical pharmacy to achieve the effects of easy-to-obtain raw materials, simple operation, and improved quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
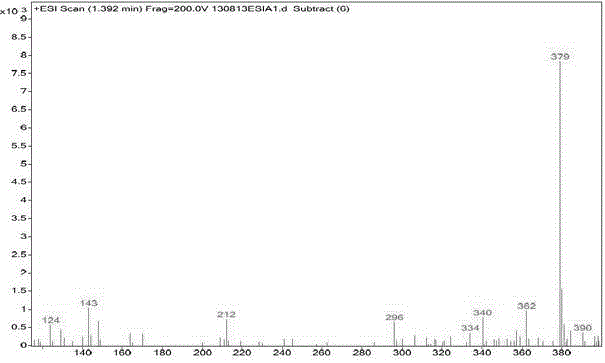
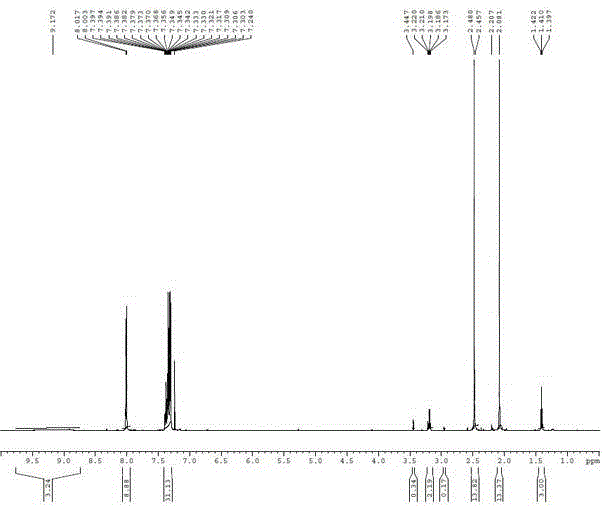
[0021] Embodiment 1: A kind of synthetic method of parecoxib sodium impurity K (N-[[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]acetamide)
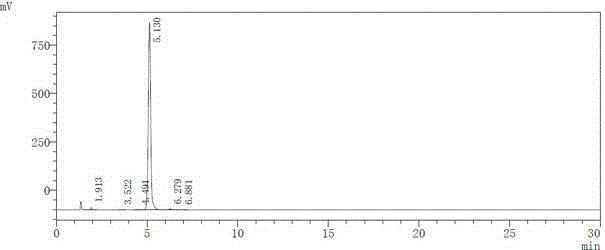
[0022] Add 20g of dichloromethane and 5g of 5-methyl-3,4-diphenylisoxazole into the reaction bottle, control the reaction temperature below 30°C, add 15g of chlorosulfonic acid dropwise, and raise the temperature to 40-50°C after the addition is complete. Reflux for 3 hours, take a sample every 0.5 hours and monitor it once with TLC (EA:PE=1:3), react until the TLC monitoring 5-methyl-3,4-diphenylisoxazole spot disappears, that is, 5-methyl-3 , 4-diphenylisoxazole reacted completely;
[0023] The reaction solution was cooled to room temperature, added dropwise to 20 g of ice water, and the temperature was controlled below 20 ° C. After the dropwise addition was completed, 15 g of dichloromethane was used to extract the liquid, and the water phase was extracted by adding 10 g of dichloromethane, and the organic phases (intermediate ...
Embodiment 2
[0028] Embodiment 2: A kind of synthetic method of parecoxib sodium impurity K (N-[[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]acetamide)
[0029] Add 20g of dichloromethane and 5g of 5-methyl-3,4-diphenylisoxazole into the reaction bottle, control the reaction temperature below 30°C, add 20g of chlorosulfonic acid dropwise, and raise the temperature to 40-50°C after the addition , refluxed for 8 hours, and monitored by TLC (EA:PE=1:3) every 0.5 hours, and reacted until the TLC monitoring 5-methyl-3,4-diphenylisoxazole spot disappeared, that is, 5-methyl- 3,4-Diphenylisoxazole reacts completely;
[0030] The reaction solution was cooled to room temperature, added dropwise to 20 g of ice water, and the temperature was controlled below 20 ° C. After the dropwise addition was completed, 15 g of dichloromethane was used to extract the liquid, and the water phase was extracted by adding 10 g of dichloromethane, and the organic phases (intermediate 1 dichloromethane solution...
Embodiment 3
[0032] Embodiment 3: A kind of synthetic method of parecoxib sodium impurity K (N-[[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonyl]acetamide)
[0033] Add 20g of dichloromethane and 5g of 5-methyl-3,4-diphenylisoxazole into the reaction flask, control the reaction temperature below 30°C, add 25g of chlorosulfonic acid dropwise, and raise the temperature to 40-50°C after the addition , refluxing for 6h, sampling every 0.5 hours and monitoring with TLC (EA:PE=1:3), and reacting until the TLC monitoring 5-methyl-3,4-diphenylisoxazole spot disappears, that is, 5-methyl- 3,4-Diphenylisoxazole reacts completely;
[0034]The reaction solution was cooled to room temperature, added dropwise to 20 g of ice water, and the temperature was controlled below 20 ° C. After the dropwise addition was completed, 15 g of dichloromethane was used to extract the liquid, and the water phase was extracted by adding 10 g of dichloromethane, and the organic phases (intermediate 1 (dichloromethane s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com