Directional preparation method and application of diaryl substituted isoxazole compound
A technology of phenylisoxazole and compounds, which is applied in the field of chemical pharmaceuticals, can solve the problems that have not been reported in the literature, and achieve the effects of improving accurate positioning and qualitative, easy control of conditions, and simple routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The preparation of embodiment 1 formula 3 compound
[0041] Mix 100g of the compound of formula 2, 300mL of concentrated sulfuric acid and 70g of anhydrous ferric chloride, mix well, and slowly add 500mL of chlorosulfonic acid dropwise at a temperature of -10°C, after the dropwise addition, stir for 0.5h; raise the temperature to 80°C for 10h; Then lower the temperature to below 30°C, slowly pour the reaction solution into 1000mL of ice-water mixture, extract with dichloromethane (500mL*2), dry the organic phase with anhydrous magnesium sulfate, filter, and concentrate the filtrate to dryness to obtain the compound containing formula 3 The product, about 80g.
Embodiment 2
[0042] The preparation of the compound of embodiment 2 formula 4
[0043] Add 400mL of dichloromethane to about 80g of the obtained product containing the compound of formula 3, lower the temperature to below 10°C, slowly add 200mL of ammonia water, keep the internal temperature at 0-5°C, after the addition is complete, keep warm for 1h; then add an appropriate amount of ammonia water , adjust the pH value of the water layer to be greater than or equal to 11; then raise the temperature to 30°C, stir and react for 10h, concentrate to remove dichloromethane, filter the obtained solid, add 200mL butanone to the obtained solid, heat to dissolve and filter, and the filtrate is in a reflux state, Slowly add 400 mL of a mixed solvent of isopropanol and water, wherein the volume ratio of isopropanol:water is 9:1, after the addition is completed, stir at 0-5°C for 8 hours, filter, and concentrate the filtrate to dryness to obtain a product containing the compound of formula 4 , about 6...
Embodiment 3
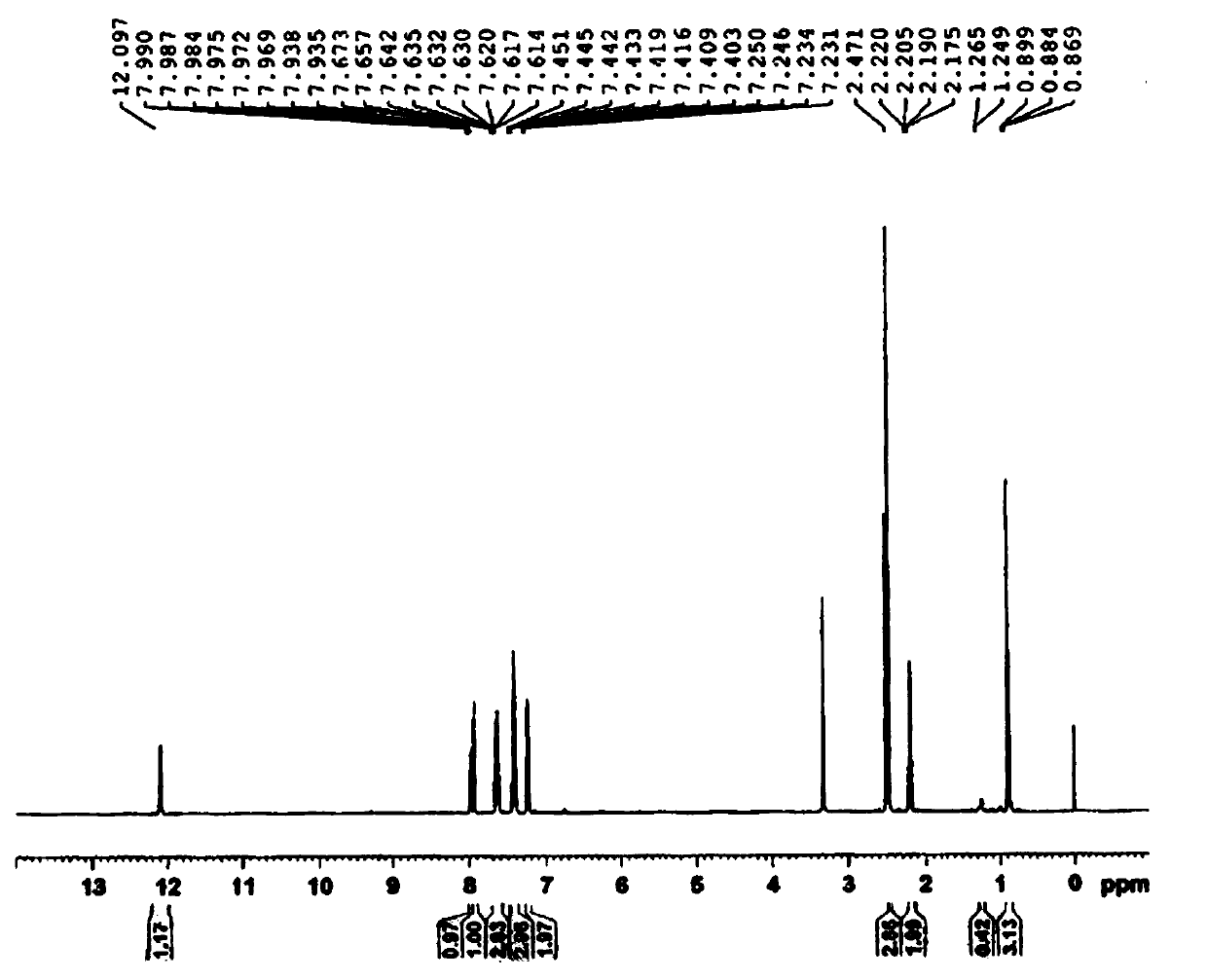
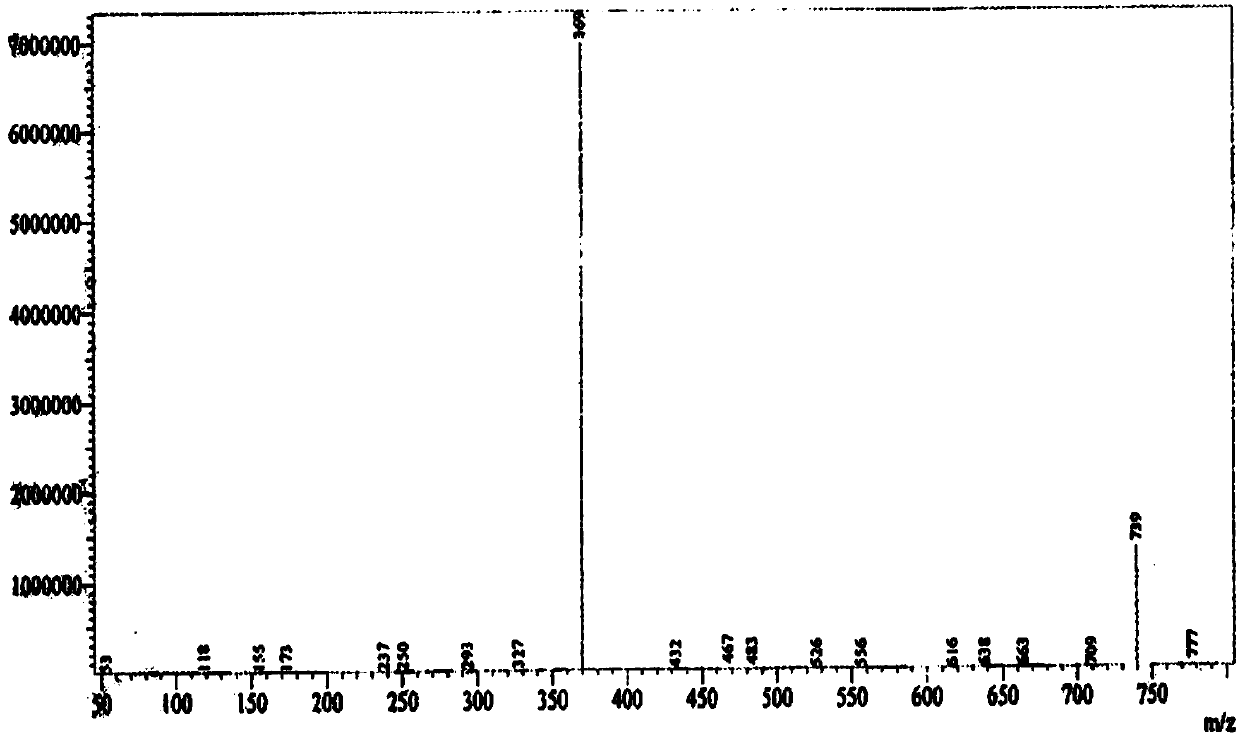
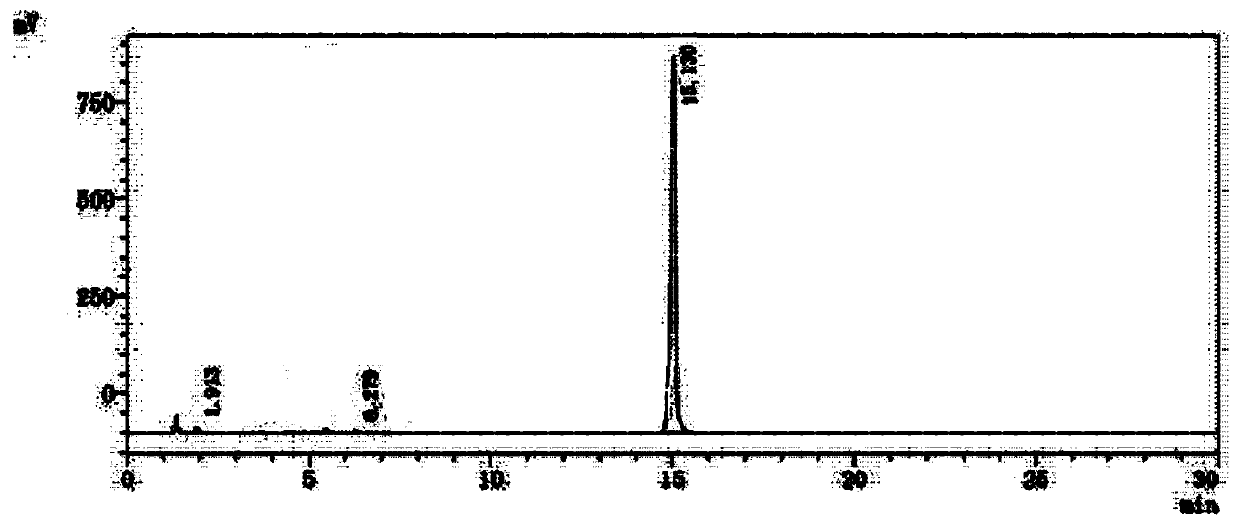
[0044] Embodiment 3 Preparation of the compound of formula 1
[0045] Add 12.8g of p-dimethylaminopyridine, 192mL of dichloromethane and 64g of triethylamine to about 62g of the obtained product containing the compound of formula 4, stir evenly, control the temperature at 10±2°C, and slowly add 64g of propionic anhydride dropwise, After the dropwise addition, react at a temperature of 25±2°C for 8 hours, monitor the completion of the reaction by TLC, cool down to 0-5°C, add 300 mL of purified water, adjust the pH of the water layer to 2-3 with hydrochloric acid solution, stir for 15 minutes, and let stand to separate layers , the organic phase was washed successively with 300 mL of purified water and 300 mL of saturated brine, dried over anhydrous magnesium sulfate, and concentrated to dryness. Add 200 mL of absolute ethanol to the obtained propionylated product, heat to 70-85°C to dissolve, and slowly add After adding 400mL of purified water, cool down to 20-30°C, stir and cr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com