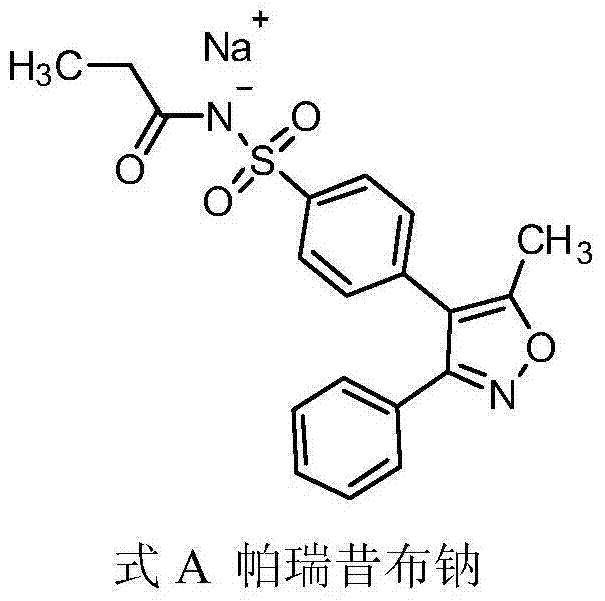
Parecoxib sodium freeze-dried powder injection and preparation method thereof
A technology of parecoxib sodium jelly and parecoxib sodium, which is applied in the field of parecoxib sodium freeze-dried powder injection, can solve the problems of large impact on the final product, many ice crystals in appearance, low solubility and the like, and achieves good Chemical stability, good reconstitution properties, and uniform solution clarification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
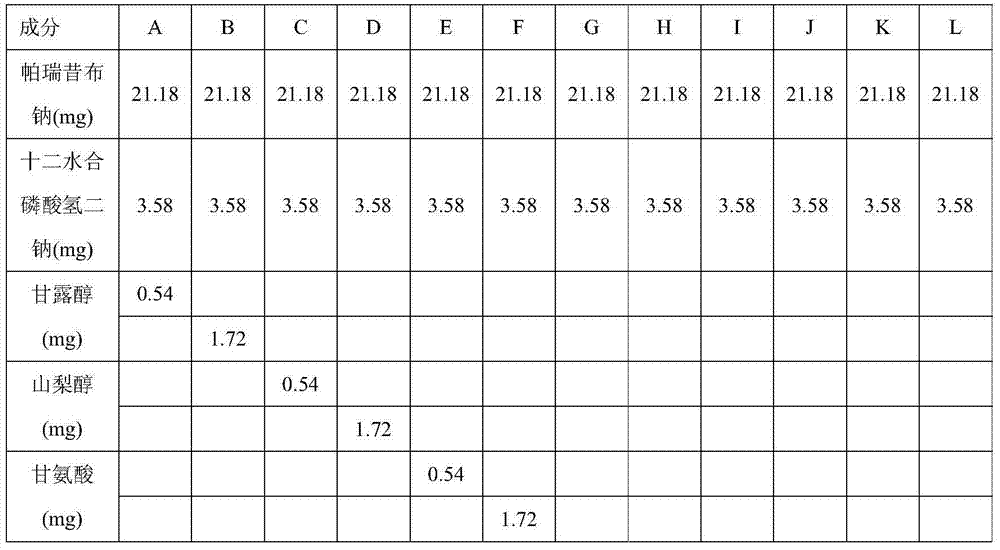
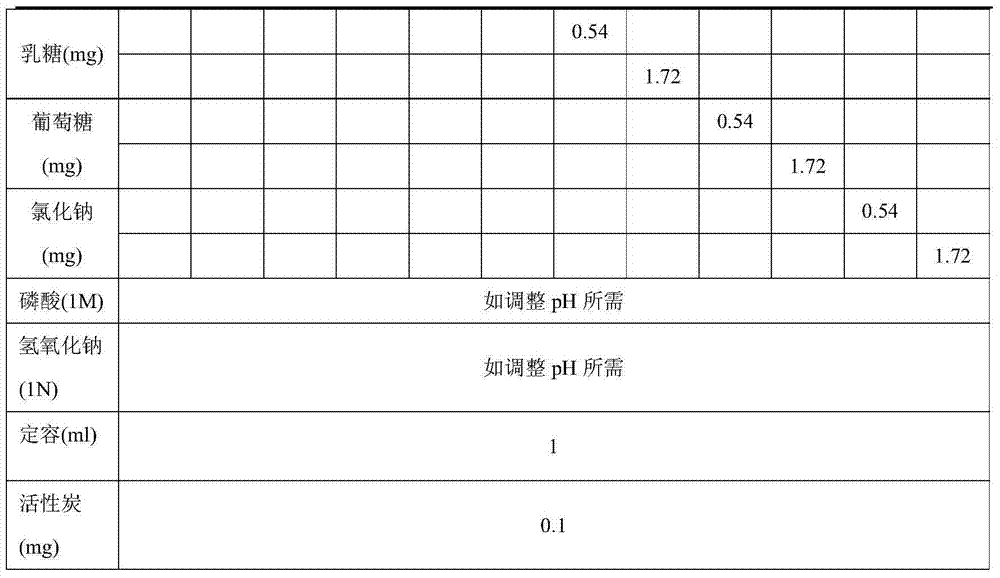
[0036] This example investigates the chemical stability of freeze-dried powder prepared with different excipients, and the content of valdecoxib is used as the index of chemical stability.
[0037] Table 1: Prescription
[0038]
[0039]
[0040] Step a dosing: Dissolve the disodium hydrogen phosphate dodecahydrate in Table 1 and the corresponding excipients of each prescription in an appropriate volume of water for injection, and if necessary, dissolve the obtained solution with 1M phosphoric acid solution or 1N sodium hydroxide solution. Adjust the pH of the solution to about 8.0-9.0, add the prescribed amount of parecoxib sodium, measure the pH, and if necessary, adjust the pH of the resulting solution to about 8.0-9.0 with 1M phosphoric acid solution or 1N sodium hydroxide solution , add water, and adjust the volume of the solution to the target volume to form a solution for lyophilization.
[0041] Step b decolorization: add the prescribed amount of activated carbo...
Embodiment 2
[0051] This example investigates the influence of the excipient sodium chloride in the freeze-dried powder injection of parecoxib sodium on the appearance and stability of the powder injection.
[0052] Table 3 Prescription
[0053]
[0054] Step a dosing: Dissolve disodium hydrogen phosphate dodecahydrate and sodium chloride corresponding to each prescription in an appropriate volume of water for injection. After completely dissolving, measure the pH. If necessary, use 1M phosphoric acid solution or 1N sodium hydroxide Solution Adjust the pH of the obtained solution to 8.0-9.0, add parecoxib sodium to dissolve in the solution, measure the pH, if necessary, adjust the pH of the obtained solution to 8.0-9.0 with 1M phosphoric acid solution or 1N sodium hydroxide solution, Water for injection was added to adjust the volume of the solution to the target volume to form a solution for lyophilization.
[0055] The method of step b and step c of Example 1 was used to prepare pare...
Embodiment 3
[0066] This example investigates the effect of the concentration of the buffering agent in the freeze-dried powder injection of parecoxib sodium on the pH of the powder injection.
[0067] Table 6: Prescription
[0068]
[0069] Dissolve disodium hydrogen phosphate dodecahydrate and sodium chloride in an appropriate volume of water for injection. After completely dissolving, adjust the pH of the resulting solution to 8.0-9.0 with 1M phosphoric acid solution or 1N sodium hydroxide solution. Parecoxib Dissolve sodium in this solution, measure the pH, if necessary, adjust the pH of the resulting solution to 8.0-9.0 with 1M phosphoric acid solution or 1N sodium hydroxide solution, add water, adjust the solution volume to the target volume, and form a solution. The method of step b and step c of Example 1 was used to prepare parecoxib sodium freeze-dried powder injection. The influence of the buffering agent in the freeze-dried powder injection of parecoxib sodium on pH and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com