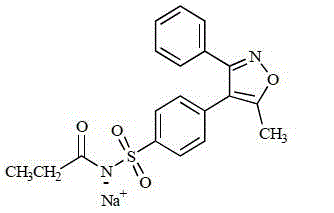
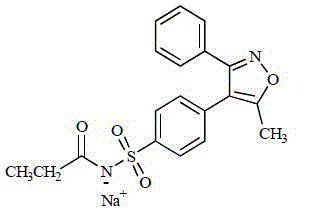
Parecoxib sodium anhydrous compound
A technology of parecoxib sodium and hydrate, which is applied in the field of parecoxib sodium anhydrous compound and its preparation, and medicine, and can solve the problem that the crystalline structure of parecoxib sodium has not been characterized.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
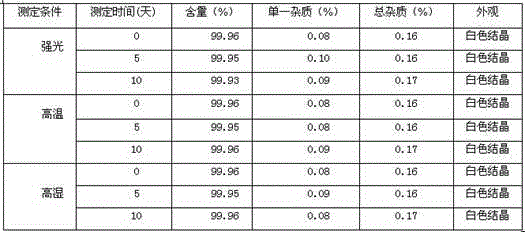
[0045] In a 500ml reaction flask equipped with a stirrer, a thermometer and a condenser, 60g of parecoxib sodium and 300ml of heptane were added, and the obtained suspension was heated to reflux for 6 hours with vigorous magnetic stirring. The crystals were collected by vacuum filtration. Vacuum drying overnight at 40°C to obtain parecoxib sodium anhydrous compound with a chemical purity of 99.9% and an optical purity of 99.96%.
[0046] Elemental analysis results:
[0047] Actual measured value (calculated value), C: 58.04 (58.10), H: 4.25 (4.33), N: 7.12 (7.14),
[0048] S: 8.21 (8.15), Na: 5.98 (5.86)
[0049] X-ray powder diffraction characteristic absorption peak (2θ) and D value are as follows:
[0050] Clef number 2θ angle (°) measured value d (?) measured value I / I0 14.5020.6175 27.2811.1381 39.139.7242 412.629.5322 512.718.32100 613.507.798 714.557.6213 814.926.8214 915.126.249 1016.445.588 1116.505.174 1217.154.831 1318.344.5726 1419.874.341 1520.104.314 1620.343.683 1720...
Embodiment 2
[0054] Injection containing parecoxib sodium anhydrate
[0055] Prescription: Parecoxib sodium anhydrate (20g based on parecoxib), 3g disodium hydrogen phosphate, 0.5g sodium dihydrogen phosphate, appropriate amount of phosphoric acid / sodium hydroxide, 2L water for injection, made into 1000 bottles.
[0056] Process: Take 90% of the prescription amount of water for injection, first add the prescription amount of disodium hydrogen phosphate and sodium dihydrogen phosphate and stir to dissolve, add 0.1 mol / L phosphoric acid or sodium hydroxide aqueous solution, adjust the pH, and then add the prescription amount to the solution Sodium parecoxib, stir to dissolve completely; add 0.1% of the solution amount of charcoal for needles, leave it for 15 minutes, filter, add water for injection to the full amount; add 0.1mol / L phosphoric acid or sodium hydroxide aqueous solution to adjust the pH , Fine filtration, quality control of intermediates, filling, semi-press stopper, put the aliquote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com