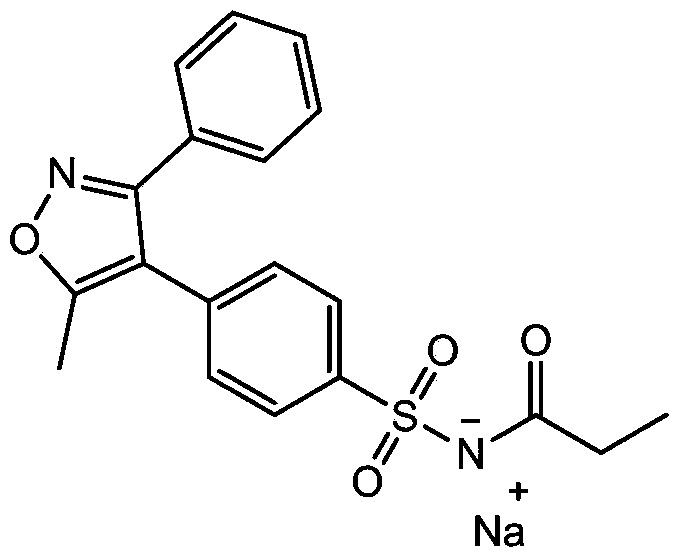
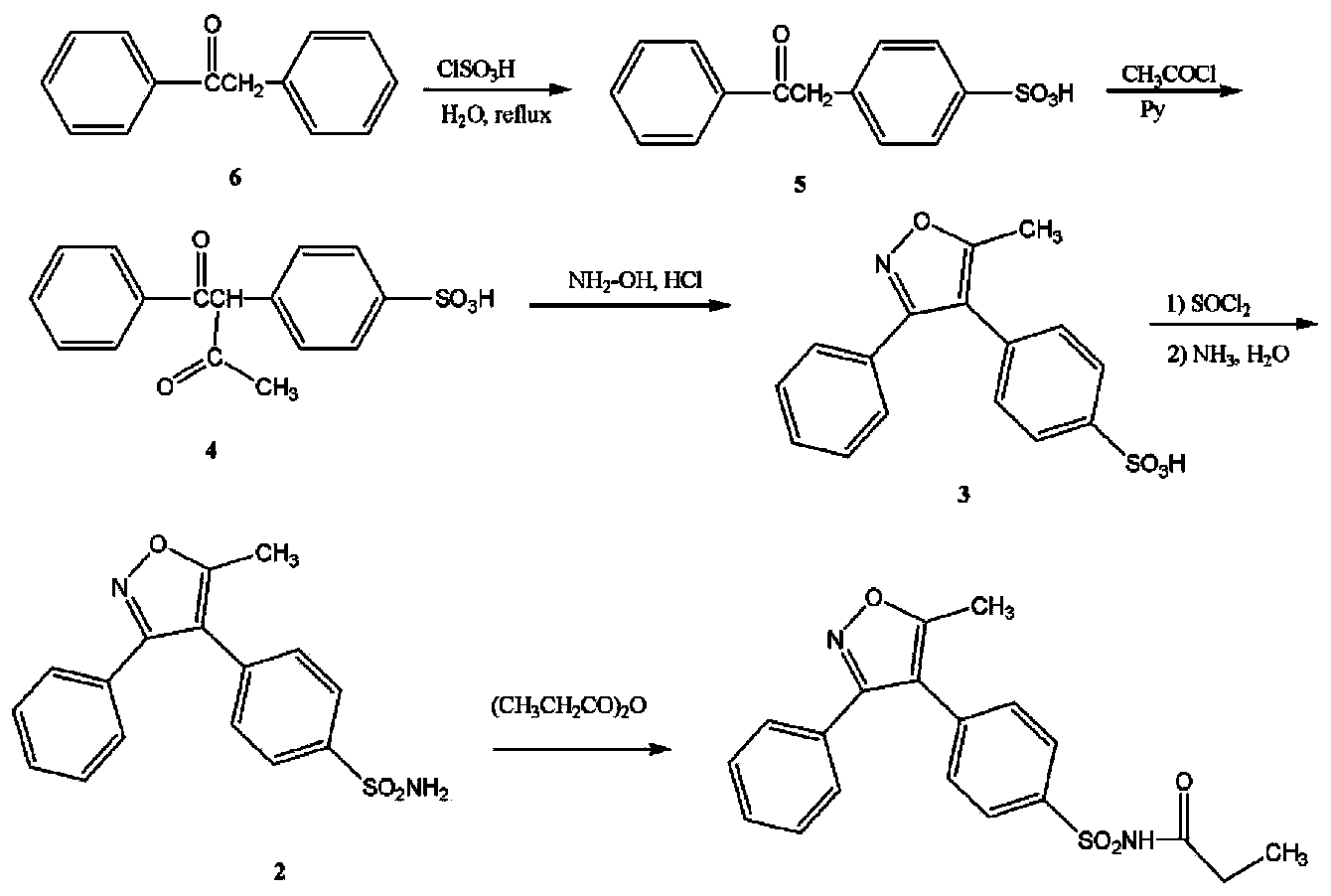
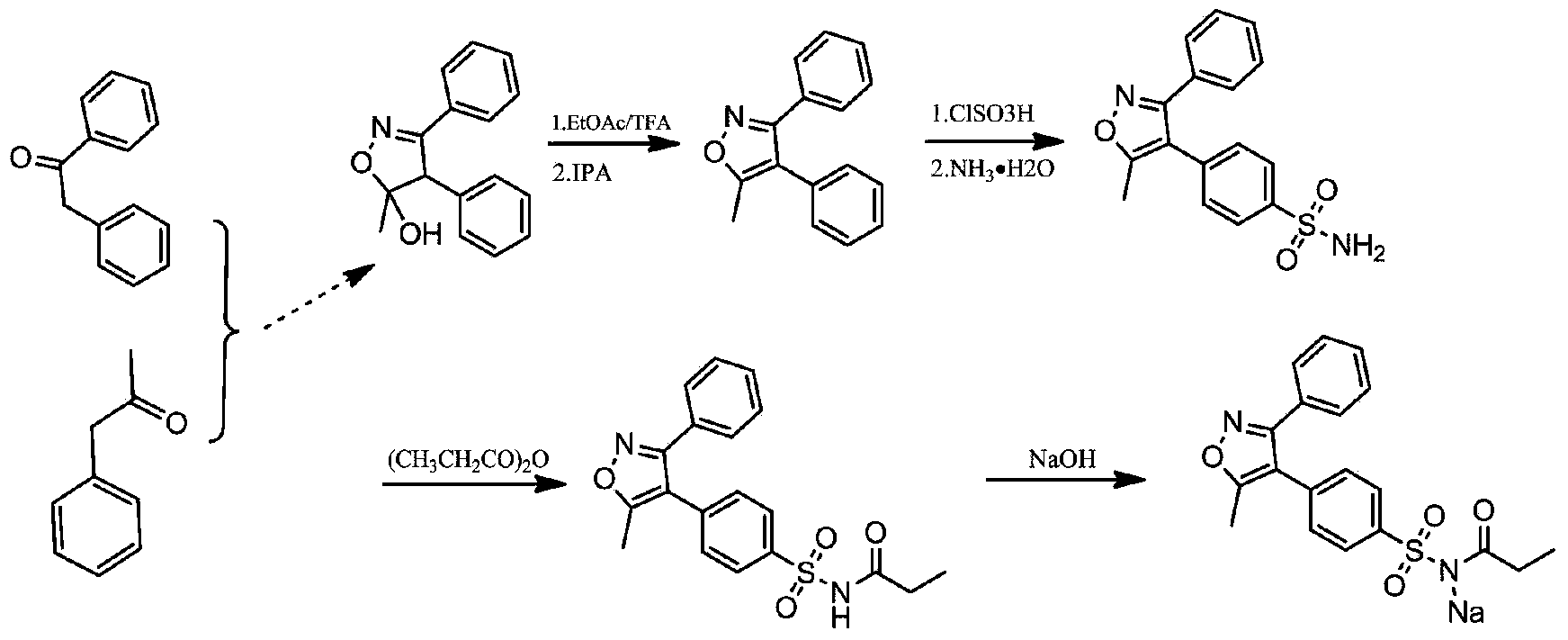
Preparation method of parecoxib sodium
A technology of parecoxib sodium and valdecoxib, which is applied in the field of medicinal chemistry, can solve the problems of low purity, high cost, and low yield, and achieve the effect of simple operation, low price, and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Dissolve 50g of 3,4-diphenyl-5-methylisoxazole in 50mL of dichloromethane, add 100g of chlorosulfonic acid, and heat to 50-60°C for reaction; liquid, the aqueous phase was extracted with dichloromethane, the aqueous layer was discarded, and the organic phases were combined to obtain a dichloromethane solution of 4-(5-methyl-3-phenyl-4-isoxazole) benzenesulfonyl chloride without passing through processing, proceed directly to the next step.
[0045] Add the dichloromethane solution of 4-(5-methyl-3-phenyl-4-isoxazole)benzenesulfonyl chloride directly into 100mL of ammonia water, react at 20-30°C, after the reaction is complete, directly concentrate under reduced pressure Remove dichloromethane, then add water to dilute, crystallize, filter, and dry, that is, about 58g of daldecoxib, which is directly carried out to the next step without further treatment.
[0046] Add 58g of valdecoxib, 58ml of propionic anhydride, and 3ml of hydrochloric acid into the reaction kettle, ...
Embodiment 2
[0051] Dissolve 50g of 3,4-diphenyl-5-methylisoxazole in 300mL of toluene, add 300g of chlorosulfonic acid, and heat to 30-40°C to react; after the reaction is complete, quench with ice water, separate the liquid, The aqueous phase was extracted with toluene, the aqueous layer was discarded, and the organic phases were combined to obtain a toluene solution of 4-(5-methyl-3-phenyl-4-isoxazole)benzenesulfonyl chloride, which was directly carried out to the next step without treatment. .
[0052] Add the toluene solution of 4-(5-methyl-3-phenyl-4-isoxazole)benzenesulfonyl chloride directly into 300mL ammonia water and react at 0-10°C. After the reaction is complete, directly concentrate the toluene under reduced pressure, Add water to dilute, precipitate solid, filter, dry, that is about 60g of daldecoxib, without further treatment, directly proceed to the next step.
[0053] Add 60g of valdecoxib and 180ml of propionic anhydride into the reaction kettle, heat to 130-140°C to re...
Embodiment 3
[0057] Dissolve 50g of 3,4-diphenyl-5-methylisoxazole in 400mL of dichloromethane, add 350g of chlorosulfonic acid, and heat to 50-60°C for reaction; liquid, the aqueous phase was extracted with dichloromethane, the aqueous layer was discarded, and the organic phases were combined to obtain a dichloromethane solution of 4-(5-methyl-3-phenyl-4-isoxazole) benzenesulfonyl chloride without passing through processing, proceed directly to the next step.
[0058] Add the dichloromethane solution of 4-(5-methyl-3-phenyl-4-isoxazole)benzenesulfonyl chloride directly into 100mL ammonia water, heat to 45-60°C to react, after the reaction is completed, directly concentrate under reduced pressure Dichloromethane was added for dilution with water, and a solid was precipitated, filtered, and dried, that is, about 62 g of devaldecoxib, which was directly carried out to the next step without further treatment.
[0059] Add 62g of valdecoxib and 124ml of methyl propionate into the reaction ket...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com