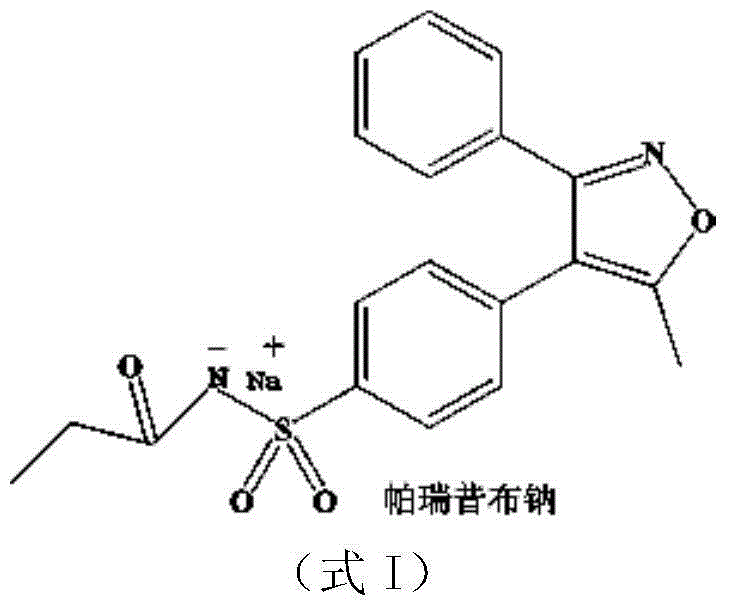
Synthesis method of parecoxib sodium
A technology of parecoxib sodium and its synthesis method, which is applied in the direction of organic chemistry, can solve the problems of increased reaction cycle, long reaction time, source limitation, etc., and achieves the effects of less environmental pollution, convenient operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
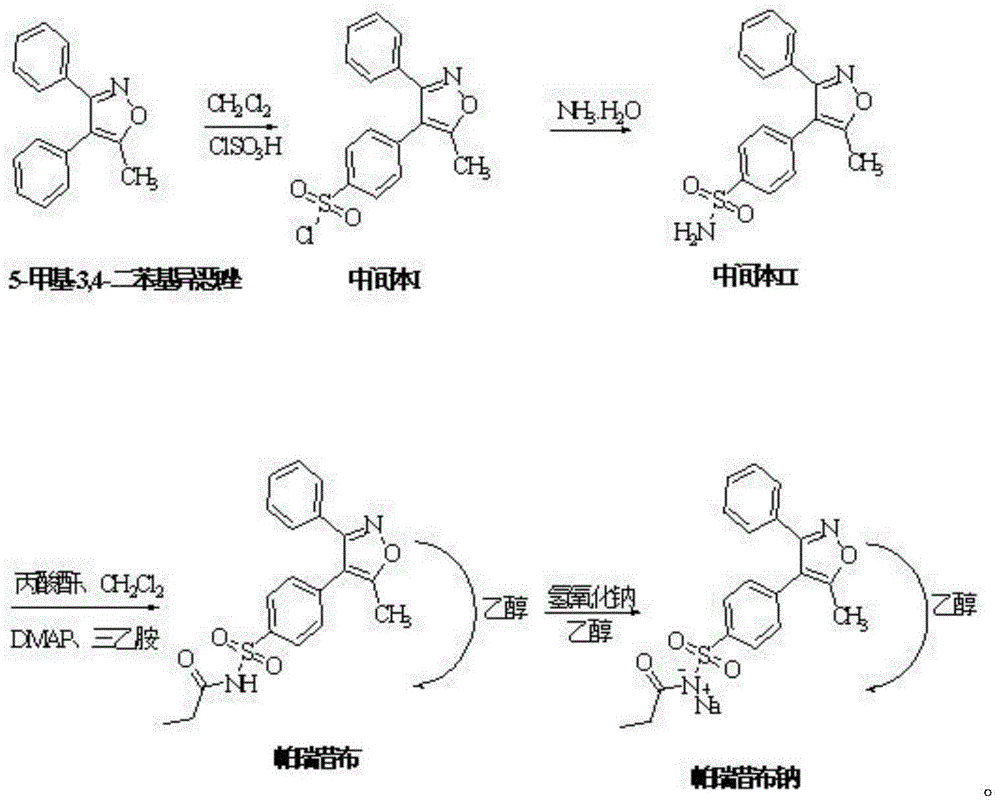
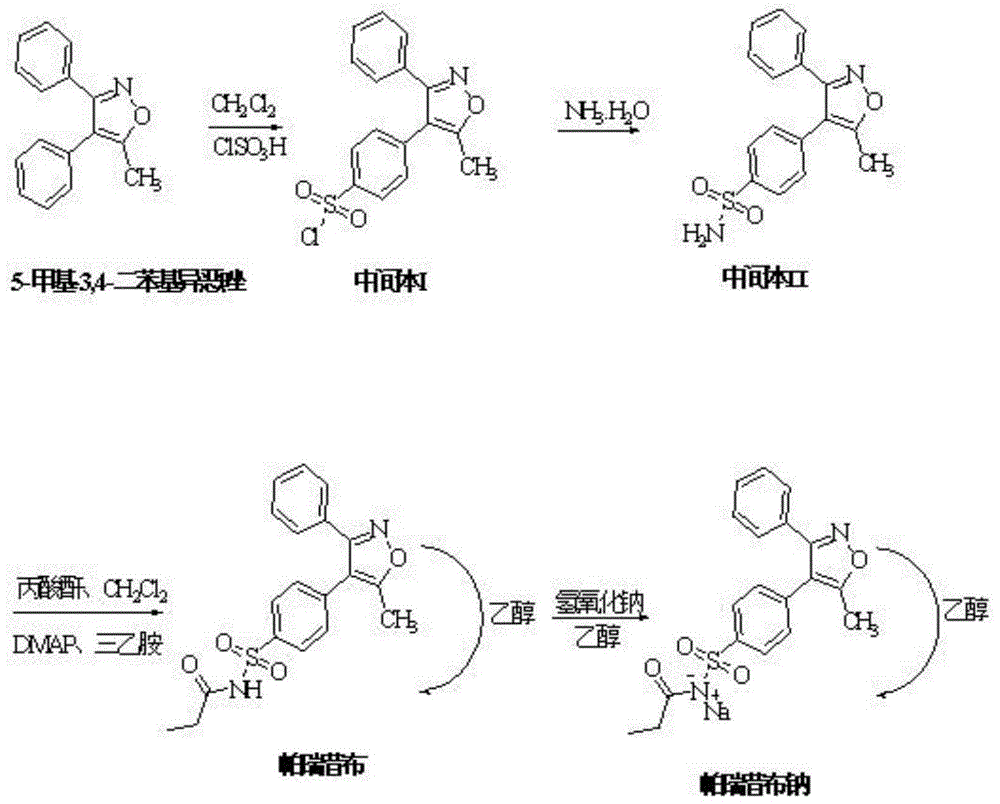
[0024] Embodiment 1: a kind of synthetic method of parecoxib sodium, it comprises the following steps:
[0025] S1. Sulfonation reaction: Add 15kg of dichloromethane and 4.5kg of 5-methyl-3,4-diphenylisoxazole into the reaction kettle, control the reaction temperature below 30°C, add 13.5kg of chlorosulfonic acid dropwise, and add After completion, heat up to 40-50°C, reflux for 3 hours, take a sample every 0.5 hours and monitor it with TLC (EA:PE=1:3), and react until the TLC monitoring 5-methyl-3,4-diphenylisoxazole spot disappears , that is, the reaction of 5-methyl-3,4-diphenylisoxazole is complete, the reaction solution is cooled to room temperature, and added dropwise to 18kg of ice water, and the temperature is controlled below 20°C. After the dropwise addition, 15kg of dichloromethane Extraction and liquid separation, add 10kg of dichloromethane to the aqueous phase for extraction, and combine the organic phases to obtain the parecoxib sodium intermediate I dichloromet...
Embodiment 2
[0029] Embodiment 2: a kind of synthetic method of parecoxib sodium, it comprises the following steps:
[0030] S1. Sulfonation reaction: add 15kg of dichloromethane and 4.5kg of 5-methyl-3,4-diphenylisoxazole into the reaction kettle, control the reaction temperature below 30°C, add 22.5kg of chlorosulfonic acid dropwise, After the addition, raise the temperature to 40-50°C, reflux for 8 hours, take samples every 0.5 hours and monitor them with TLC (EA:PE=1:3), and monitor the spots of 5-methyl-3,4-diphenylisoxazole by TLC after reaction disappear, that is, the reaction of 5-methyl-3,4-diphenylisoxazole is complete, the reaction solution is cooled to room temperature, added dropwise to 18kg ice water, and the temperature is controlled below 20°C. After the dropwise addition, 15kg dichloro Methane extraction and liquid separation, adding 10 kg of dichloromethane to the aqueous phase for extraction, and combining the organic phases to obtain the parecoxib sodium intermediate I ...
Embodiment 3
[0034] Embodiment 3: a kind of synthetic method of parecoxib sodium, it comprises the following steps:
[0035] S1. Sulfonation reaction: Add 15kg of dichloromethane and 4.5kg of 5-methyl-3,4-diphenylisoxazole into the reaction kettle, control the reaction temperature below 30°C, add 18kg of chlorosulfonic acid dropwise, add dropwise After completion, heat up to 40-50°C, reflux for 5 hours, take a sample every 0.5 hours and monitor it once with TLC (EA:PE=1:3), and react until the TLC monitoring 5-methyl-3,4-diphenylisoxazole spot disappears , that is, the reaction of 5-methyl-3,4-diphenylisoxazole is complete, the reaction solution is cooled to room temperature, and added dropwise to 18kg of ice water, and the temperature is controlled below 20°C. After the dropwise addition, 15kg of dichloromethane Extraction and liquid separation, add 10kg of dichloromethane to the aqueous phase for extraction, and combine the organic phases to obtain the parecoxib sodium intermediate I dic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com