Method for detecting parecoxib sodium sulfate genotoxic impurities
A technology of parecoxib sodium and a detection method, which is applied in the detection field of parecoxib sodium sulfate genotoxic impurities, can solve the problem that no reports of dimethyl sulfate diisopropyl sulfate and no simultaneous detection of sulfuric acid have been found. Ester impurities and other problems, to achieve the effect of high detection sensitivity, easy operation, high specificity and durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 1) Preparation of solution
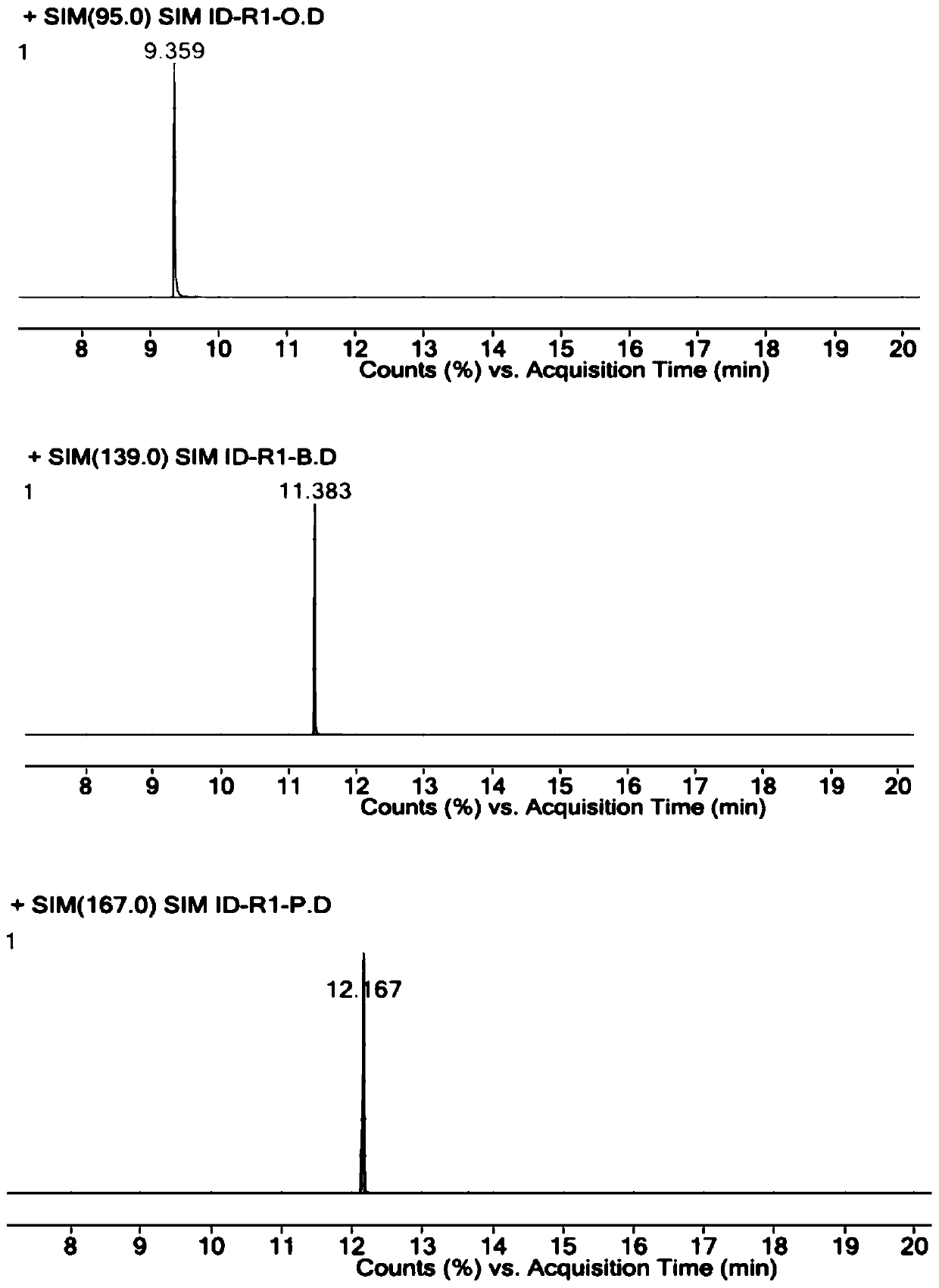
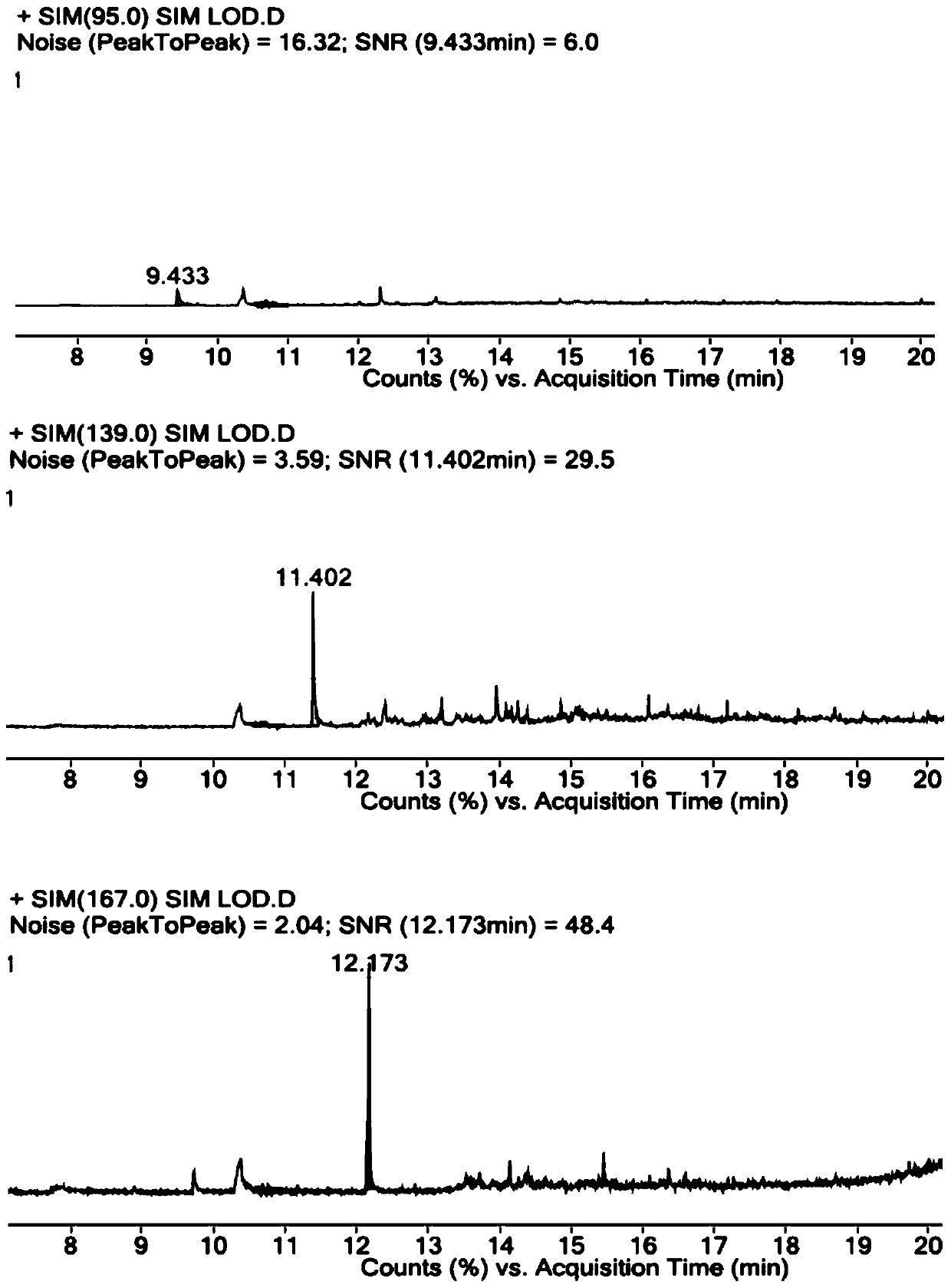
[0058] Accurately weigh R1-O reference substance, R1-B reference substance, R1-P reference substance and parecoxib sodium reference substance, add DMSO, dissolve and dilute to the concentration of R1-O reference substance is 1 μg / ml, R1- The concentration of the B reference substance is 1 μg / ml, the concentration of the R1-P reference substance is 1 μg / ml, and the concentration of the parecoxib sodium reference substance is 25 mg / ml, which is used as the resolution solution.
[0059] 2) Instruments and testing conditions:
[0060] Instrument: Agilent5975B MSD in series with 6890GC
[0061] Chromatographic column: gas chromatography column SE-54
[0062] Constant flow mode, the carrier gas is helium, the auxiliary gas is hydrogen and air, the carrier gas flow rate: 1ml / min.
[0063] Column temperature: heating program, the initial temperature is 40°C, the temperature continues to rise to 300°C, and the temperature of the injection port is ...
Embodiment 2
[0071] 1) Preparation of solution
[0072] Accurately weigh R1-O reference substance, R1-B reference substance, R1-P reference substance and parecoxib sodium reference substance, add methanol, dissolve and dilute to the concentration of R1-O reference substance is 1 μg / ml, R1- The concentration of the B reference substance is 1 μg / ml, the concentration of the R1-P reference substance is 1 μg / ml, and the concentration of the parecoxib sodium reference substance is 25 mg / ml, which is used as the resolution solution.
[0073] 2) Instruments and testing conditions:
[0074] Instrument: Agilent5975B MSD in series with 6890GC
[0075] Chromatographic column: gas chromatography column DB-624
[0076] Constant flow mode, the carrier gas is helium, the auxiliary gas is hydrogen and air, the carrier gas flow rate: 1ml / min.
[0077] Column temperature: heating program, the initial temperature is 40°C, the temperature continues to rise to 300°C, and the temperature of the injection por...
Embodiment 3
[0085] 1) Preparation of solution
[0086] Accurately weigh R1-O reference substance, R1-B reference substance, R1-P reference substance and parecoxib sodium reference substance, add tetrahydrofuran, dissolve and dilute to the concentration of R1-O reference substance is 1 μg / ml, R1- The concentration of the B reference substance is 1 μg / ml, the concentration of the R1-P reference substance is 1 μg / ml, and the concentration of the parecoxib sodium reference substance is 1 mg / ml, which is used as the resolution solution.
[0087] 2) Instruments and testing conditions:
[0088] Instrument: Agilent5975B MSD in series with 6890GC
[0089] Chromatographic column: gas chromatography column DB-5
[0090] Constant flow mode, the carrier gas is helium, the auxiliary gas is hydrogen and air, the carrier gas flow rate: 5ml / min.
[0091] Column temperature: heating program, the initial temperature is 60°C, the temperature continues to rise to 350°C, and the temperature of the injection...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com