Synthesis method of parecoxib sodium impurity
A technology of parecoxib sodium and its synthesis method, which is applied in the field of chemical pharmacy to achieve the effects of cheap raw materials, simple operation and improved quality of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Synthesis of Parecoxib Sodium Impurity E N-[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonic acid
[0023] Add 3g of 5-methyl-3,4-diphenylisoxazole, 9g of dichloromethane, and 6g of chlorosulfonic acid into the reaction flask, reflux at 40°C for 4 hours, add the reaction solution dropwise to 50g of water, and add 12g of dichlorosulfonic acid Extracted with methyl chloride, concentrated to dryness, added 3g of ethyl acetate, crystallized 12g of petroleum ether for 1.5 hours, and filtered to obtain 3.24g of intermediate I, with a yield of 76.1%.
[0024] Add 2g of intermediate I to the reaction flask, add 4g of water, 4g of acetonitrile, reflux at 85°C for 12h, reduce the pressure to -0.07MPa and concentrate to obtain 1.78g of white solid, with a yield of 94.2%.
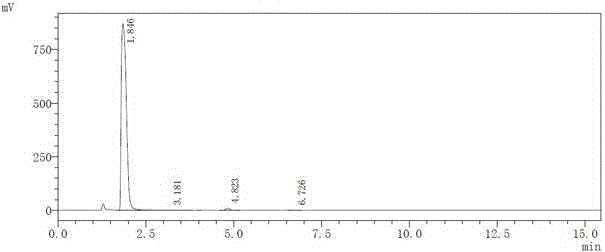
[0025] Parecoxib sodium impurity E purity detection HPLC spectrum is as follows figure 1 As shown, the purity is 99.0%. ;
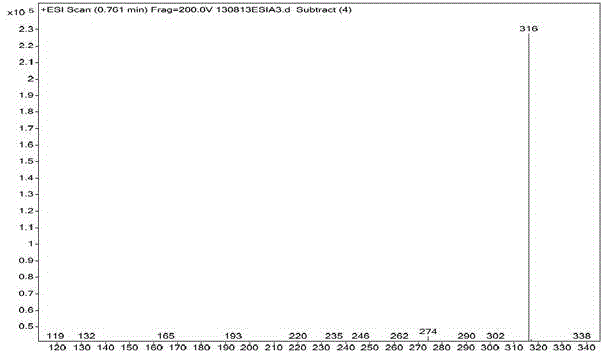
[0026] Parecoxib Sodium Impurity E Mass Spectrum figure 2 As shown, MS...
Embodiment 2
[0028] Example 2: Synthesis of Parecoxib Sodium Impurity E N-[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonic acid
[0029] Add 3g of 5-methyl-3,4-diphenylisoxazole, 18g of dichloromethane, and 15g of chlorosulfonic acid into the reaction flask, reflux at 50°C for 8 hours, add the reaction solution dropwise to 50g of water, and add 12g of dichlorosulfonic acid Extract with methyl chloride, concentrate to dryness, add 3g of ethyl acetate, crystallize 12g of petroleum ether for 2.5 hours, filter to obtain 3.17g of intermediate I, yield 74.5%.
[0030] Add 2g of intermediate I to the reaction flask, add 10g of water, 10g of acetonitrile, reflux at 95°C for 20h, depressurize to -0.08MPa and concentrate to obtain 1.82g of white solid, yield 96.3%, HPLC purity 98.7%.
Embodiment 3
[0031] Example 3: Synthesis of Parecoxib Sodium Impurity E N-[4-(5-methyl-3-phenyl-4-isoxazolyl)phenyl]sulfonic acid
[0032] Add 5g of 5-methyl-3,4-diphenylisoxazole, 20g of dichloromethane, and 20g of chlorosulfonic acid into the reaction flask, reflux at 42°C for 6 hours, add the reaction solution dropwise to 85g of water, and add 20g of dichlorosulfonic acid Extract with methyl chloride, concentrate to dryness, add 5g of ethyl acetate, crystallize 20g of petroleum ether for 2 hours, and filter to obtain 5.80g of intermediate I, with a yield of 81.7%.
[0033] Add 5g of intermediate I to the reaction flask, add 15g of water, 15g of acetonitrile, reflux at 90°C for 16h, depressurize to -0.10MPa and concentrate to obtain 4.45g of white solid, yield 94.2%, HPLC purity 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com