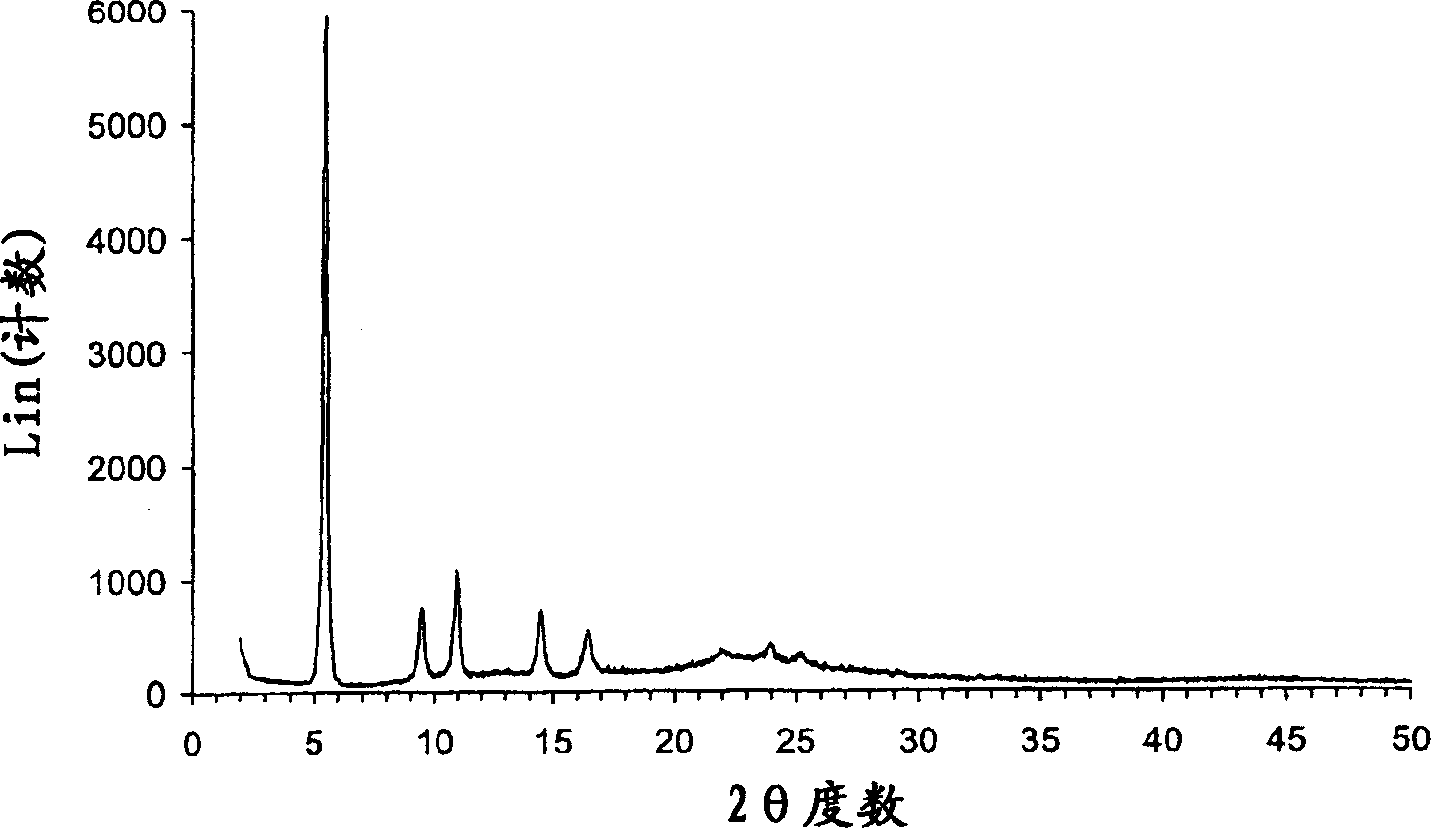
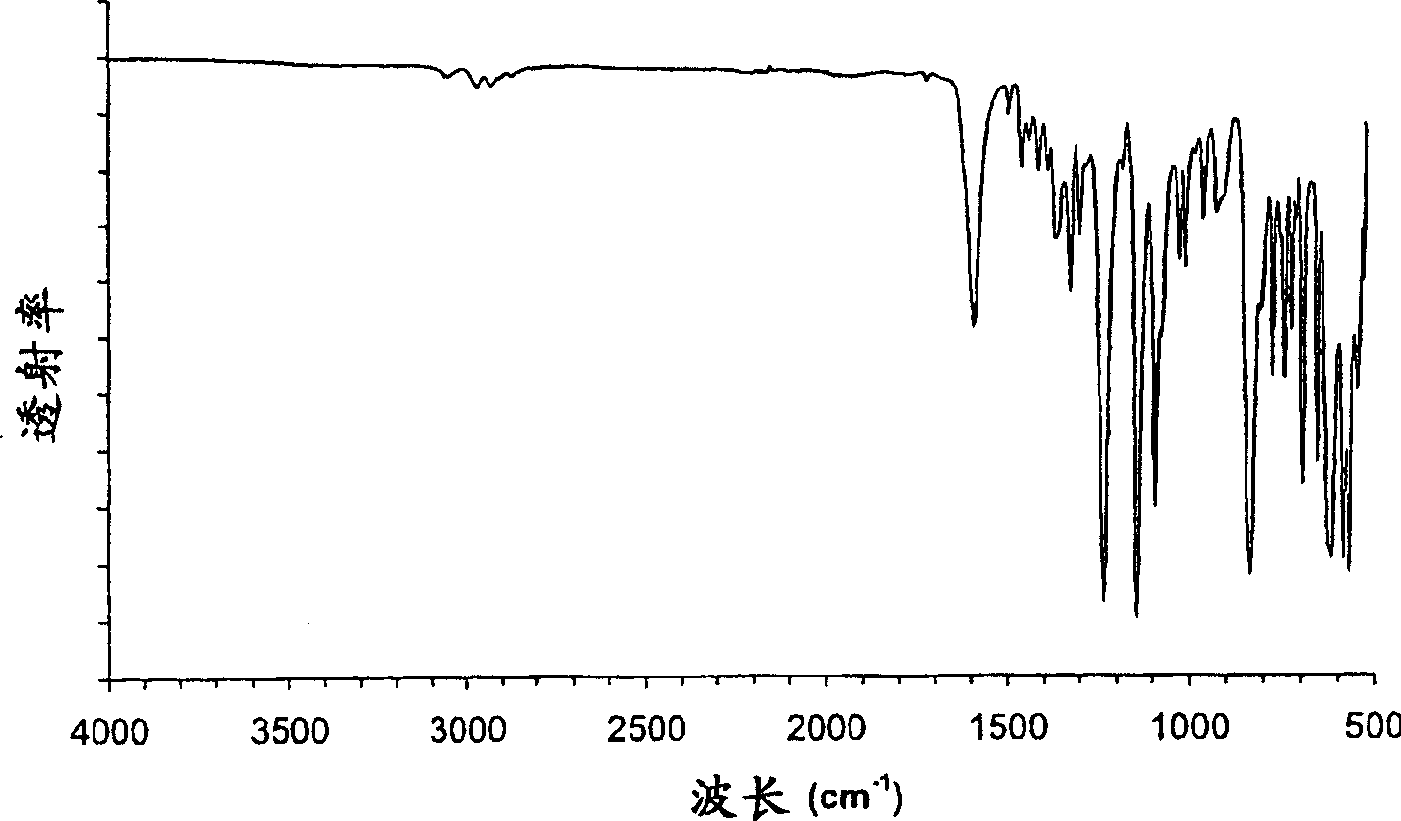
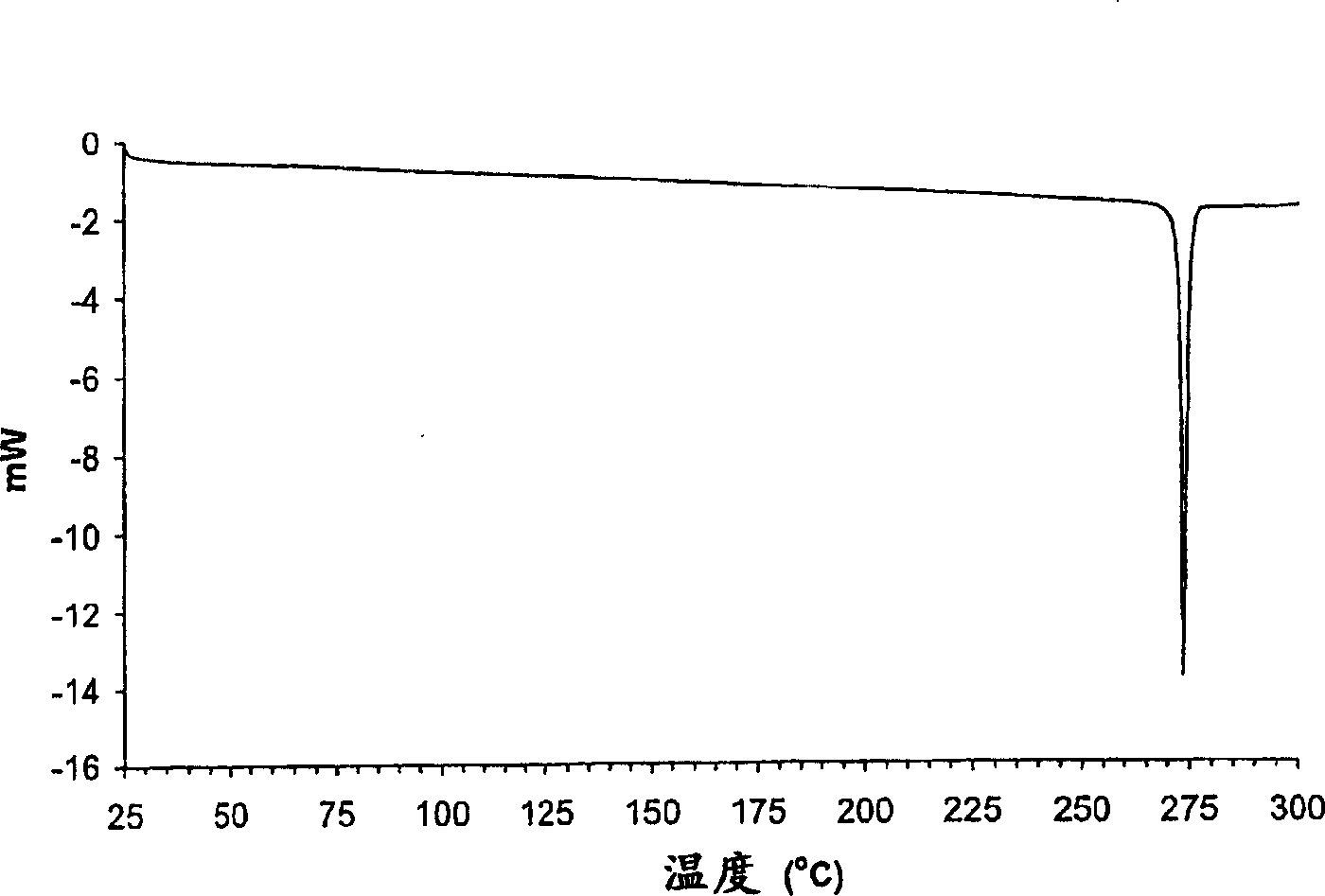
Crystalline parecoxib sodium
A parecoxib sodium, crystallization technology, applied in the field of parecoxib sodium crystal form, can solve the problem of weak inhibitory activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] Preparation of Parecoxib Sodium
[0053] The parecoxib sodium that can be used in the preparation of any of the above anhydrous, non-solvated crystalline forms or any of the pharmaceutical products of parecoxib sodium can be prepared by any suitable method, including methods known per se. In one such approach, the synthesis of parecoxib sodium (III) involves five chemical steps starting from commercially available starting materials, as shown in Scheme 1 below.
[0054] plan 1
[0055]
[0056]
[0057] In a first step, 210 kg of deoxygenated benzoin (IV), 711 liters of ethanol, and 77 liters of 80% aqueous acetic acid were added to a reaction vessel. Alternatively, glacial acetic acid (63 L) and water (16.5 L) can be used. The mixture was heated to 70°C and 71 liters of 50% aqueous hydroxylamine and 55 liters of water were added. The mixture was kept at 70°C for at least 1 hour. In-process checks were performed to ensure that the a...
Embodiment 1
[0128] Example 1: Preparation of Form A
[0129] Parecoxib sodium Form A was prepared by each of the following methods.
[0130] 1. Lyophilize the aqueous solution of parecoxib sodium. The obtained amorphous parecoxib sodium was placed in the DSC pan in the absence of moisture and the temperature was increased at a rate of 10 °C / min. Crystallization of parecoxib sodium occurs at approximately 125-130°C and is an exothermic event. The crystals were identified as Form A by one or more of PXRD, FTIR, DSC and moisture absorption as described below.
[0131] 2. A total of 10 g of the mixture of parecoxib sodium Form A and ethanol solvate was placed in an oven at 150°C for 3 hours at ambient pressure. The resulting solid was cooled in a desiccant bottle containing Drierite desiccant and confirmed to be Form A by one or more of PXRD, FTIR, DSC and moisture absorption as described below.
[0132] 3. Form E parecoxib sodium was found to convert to Form A at approximately 210° C. ...
Embodiment 2
[0134] Example 2: Preparation of Form B
[0135] Parecoxib sodium Form B was prepared by each of the following methods.
[0136] 1. Expose Parecoxib Sodium Form A to about 75% RH for several days to produce a hydrated crystalline form. This hydrated crystalline form is then dried over a desiccant. The obtained solid was identified as Form B by one or more of PXRD, FTIR, DSC and moisture absorption as described below.
[0137] 2. The ethanol solvate of Parecoxib Sodium was prepared by recrystallizing 11.5 g of Parecoxib Sodium in 100 ml of ethanol by heating to boiling on a hot plate with magnetic stirring, followed by ambient cooling to room temperature. Separately, about 1 g of Form B seeds were added to 450 ml of heptane. The freshly prepared ethanol solvate was collected by vacuum filtration and immediately transferred into a heptane suspension containing Form B seeds. The obtained suspension was heated to reflux for 4 hours with vigorous magnetic stirring. Crystals...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com