Plectasin expressed through bacillus subtilis and expressing method thereof
A technology of Bacillus subtilis and mycelia, applied in chemical instruments and methods, antibacterial drugs, pharmaceutical formulations, etc., can solve problems such as difficulty, high cost of chemical synthesis, and inability to form large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Gene Synthesis
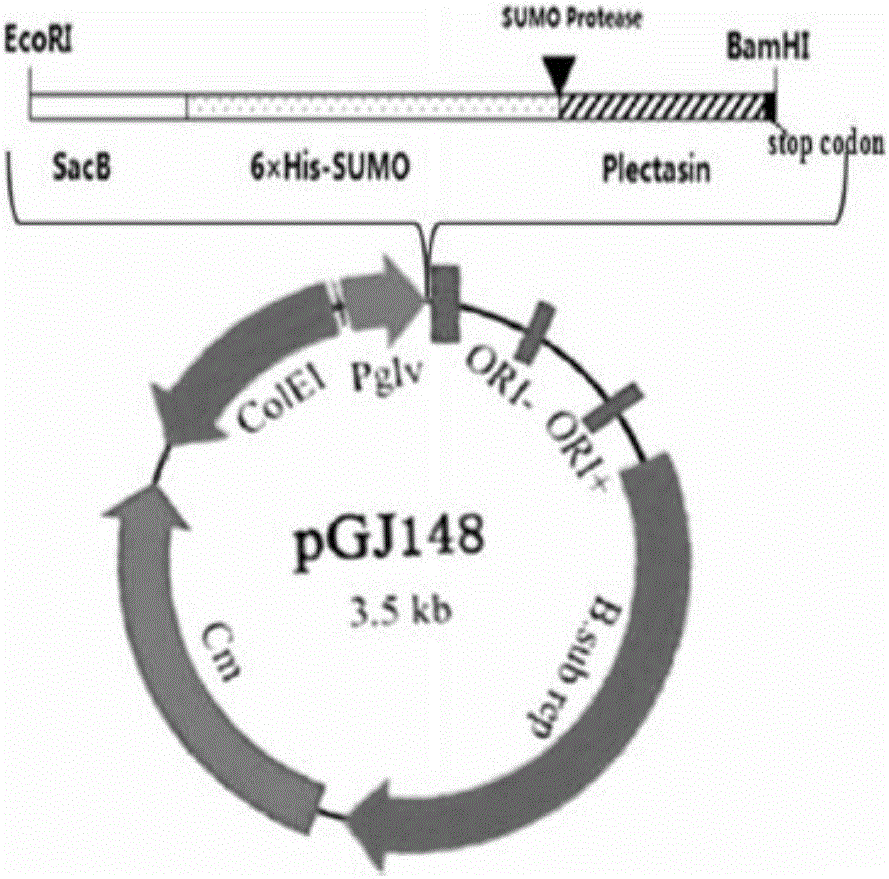
[0024] Whole-gene synthesis of the plectasin gene with signal peptide SacB and protein tag 6×His-SUMO, and two enzyme cutting sites EcoRI and BamHI were designed at both ends of the gene. The gene sequence is as follows: figure 1 shown. Gene synthesis was completed by Shanghai Sangon Bioengineering Co., Ltd.
Embodiment 2
[0025] Example 2 Construction of pGJ148-Plectasin expression plasmid
[0026] The gene synthesized in Example 1 and the shuttle vector pGJ148 were double-digested with EcoRI and BamHI respectively, and after recovery from the gel, they were treated with T4 ligase overnight at 4°C. The ligation product was transformed into Escherichia coli competent cell DH5α and the plasmid was extracted. The recombinant plasmid structure is as figure 2 shown. The correct recombinant plasmid pGJ148-Plectasin verified by PCR, double enzyme digestion and sequencing was electrotransformed into the host expression bacteria Bacillus subtilis WB800N.
Embodiment 3
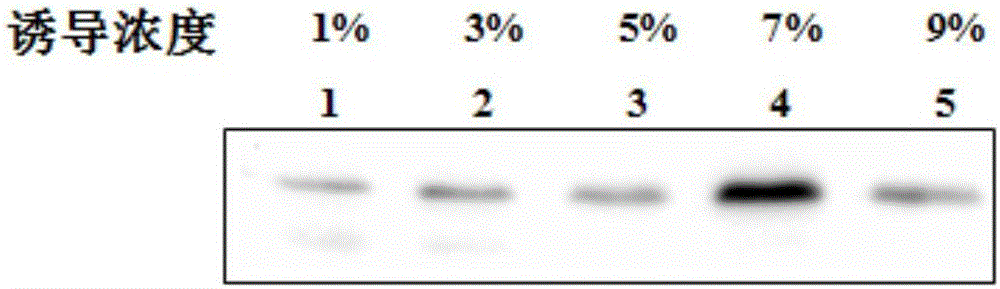
[0027] Example 3 Recombinant strain vial-induced expression
[0028] Pick the above-mentioned recombinant strain WB800N-pGJ148-Plectasin in 10mL LB medium, add neomycin and chloramphenicol so that the final concentration is 10μg / mL, culture overnight at 37°C, 220r / m, transfer to 150mL LB culture After culturing for 4 hours, add maltose for induction, so that the final concentration of maltose is 1%, 3%, 5%, 7%, 9%. After 48 hours of culture, the supernatant of the bacterial liquid was collected for Western-blot analysis. Maltose at various concentrations could induce the expression of the fusion protein. As the concentration of maltose increased, the expression of the fusion protein also increased. When , the expression of the fusion protein reached the highest level. But when the concentration of maltose reached 9%, the expression of fusion protein decreased.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com