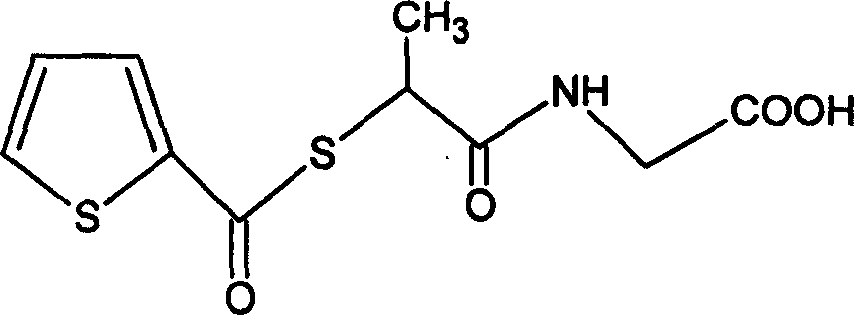
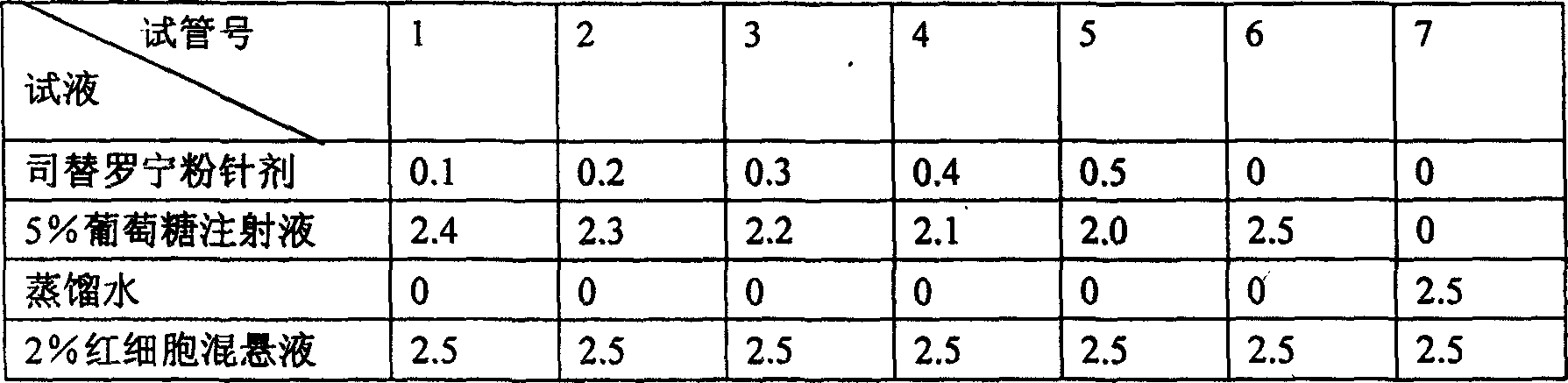
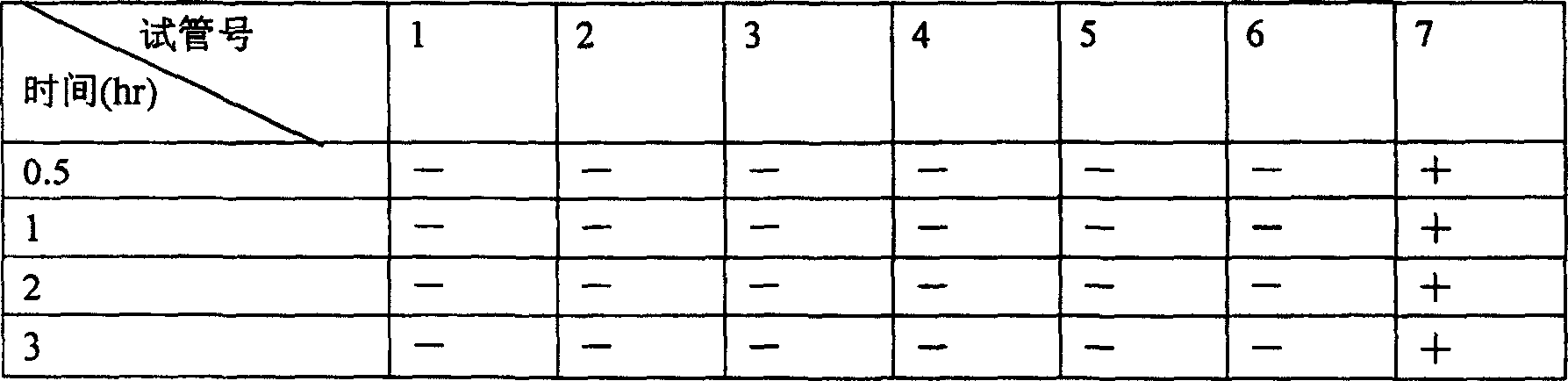
Stepronin powder injection and its preparing method
A technology of stilonine and powder injection, which is applied in the field of new dosage forms of stilonine, can solve the problems of low bioavailability, slow onset, slow absorption and distribution, etc., and achieve high bioavailability, fast distribution, and fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of steironine powder injection
[0061] 1. According to French Patent Fr 2483419, 04, the technical scheme disclosed by Dec1981 takes tiopronin as raw material to synthesize stironine;
[0062] 2. Prepare raw materials according to the following prescription:
[0063] Ingredient Amount
[0064] Steironine 200g
[0065] Mannitol 50g
[0066] Water for injection 2000ml
[0067] 3. Preparation process:
[0068] Take 200g of steironine and 50g of mannitol, add 1600ml of water for injection to dissolve, add an appropriate amount of activated carbon for needles, stir, filter, adjust the pH value to 2.0-4.0 with 0.1mol / L HCl and 0.1mol / L NaOH, add water for injection to 2000ml. Sterilize the medicinal liquid according to the aseptic operation method, filter it with a 0.22μ microporous membrane, send the filtrate sample to check the content of the semi-finished product, and then divide it into 1000 tubes according to the same amount, and pre-freeze the divide...
Embodiment 2
[0071] Preparation of steironine powder injection
[0072] Ingredient Amount
[0073] Steironine 200g
[0074] Sorbitol 50g
[0075] Add water for injection to 2000ml
[0076] A total of 1000 freeze-dried products were made
[0077] Preparation Process:
[0078] The preparation method is the same as in Example 1 except that the auxiliary materials added are different.
Embodiment 3
[0080] Steironine Sodium for Injection
[0081] Ingredient Amount
[0082] Steironine Sodium 216g
[0083] Mannitol 50g
[0084] Add water for injection to 2000ml
[0085] A total of 1000 freeze-dried products were made
[0086] Preparation Process:
[0087] The preparation method is the same as in Example 1 except that the raw materials added are different and the pH is adjusted at 7.0-9.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com