Antimicrobial peptide Pc-CATH1 and gene thereof, chemical synthesis method and application thereof
An antimicrobial peptide, pc-cath1 technology, applied in the field of biomedicine, achieves the effect of super strong serum stability, small molecular weight, and rapid bactericidal action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Cloning and gene sequencing of antimicrobial peptide Pc-CATH1 precursor gene, including:
[0036] The RNeasy Mini Kit was used to extract the total RNA from the bone marrow of the ring-necked pheasant, and the Creator TM SMART TM The cDNA library kit was used to construct the cDNA library of ring-necked pheasant bone marrow. Reverse Transcriptase reverse transcription synthesizes the first strand of cDNA, the primers are:
[0037] Forward Oligo dT primer: 5′-AAGCAGTGGTATCAACGCAGAGTGGCCATTACGGCCGGG-3′
[0038] Reverse CDSIII primer: 5′-ATTCTAGAGGCCGAGGCGGCCGACATGT(30)N -1 N-3' (N=A, G, C, or T; N -1 = A, G, or C).
[0039] The second strand was synthesized by Advantage DNA Polymerase, the primer was: forward 5′-AAGCAGTGGTATCAACGCAGAGT-3′, and the reverse primer was CDSIII.
[0040] Two specific forward primers (P1, P2) and one reverse non-specific universal primer (CDSIII) were designed, and the cDNA of cathelicidin was amplified by semi-nested PCR using the cDN...
Embodiment 2
[0104] The chemical synthesis method of Pc-CATH1:
[0105] 1. According to the amino acid sequence of the mature peptide Pc-CATH1 deduced from the gene encoding ring-necked pheasant cathelicidin, its full sequence was synthesized with an automatic polypeptide synthesizer (Applied Biosystems), and purified by desalting and desalting by HPLC reverse-phase column chromatography.
[0106] II. Molecular weight was determined by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF).
Embodiment 3
[0108] Pharmacological experiments of antimicrobial peptide Pc-CATH1:
[0109] 1. Detection of antibacterial activity of Pc-CATH1:
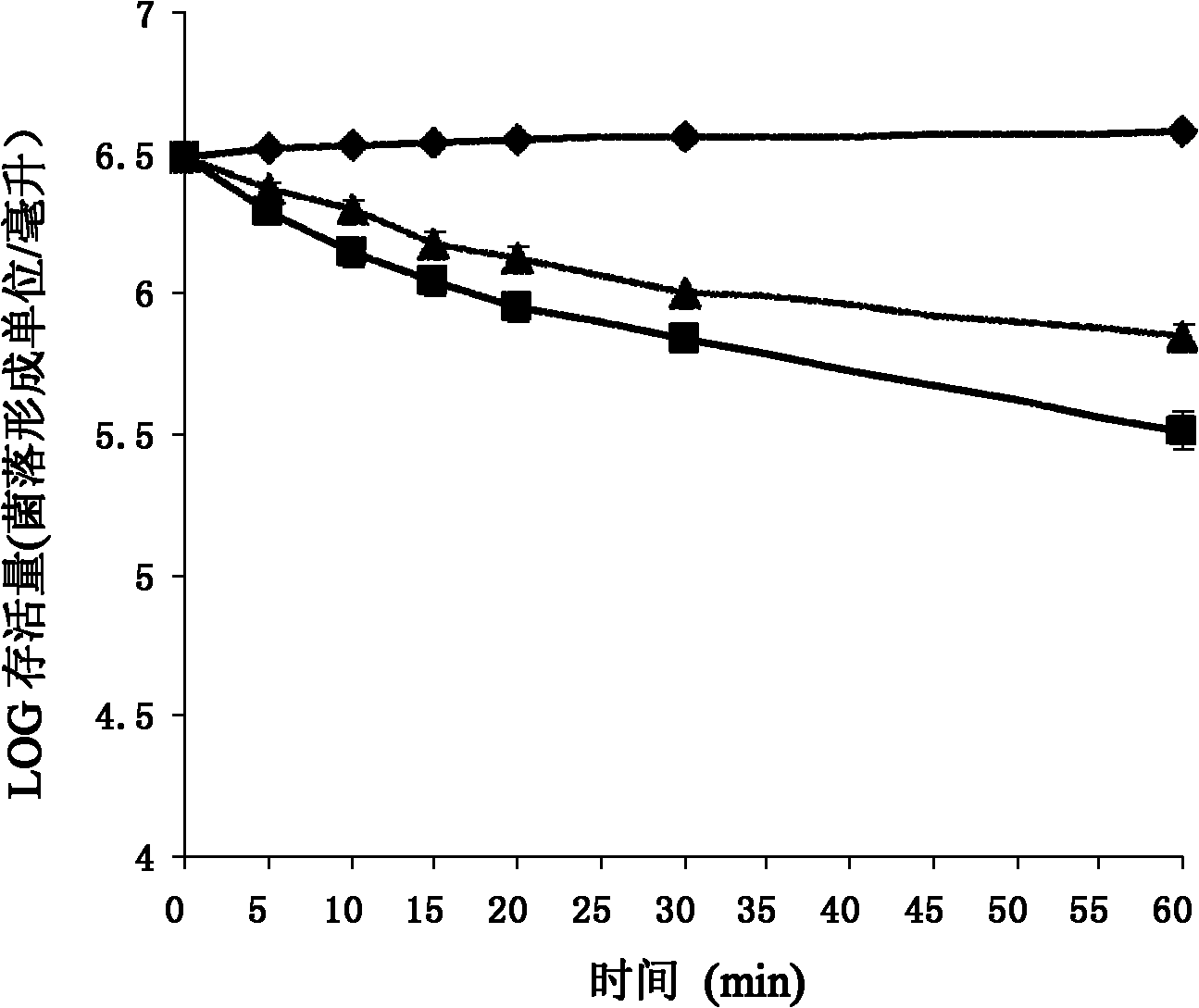
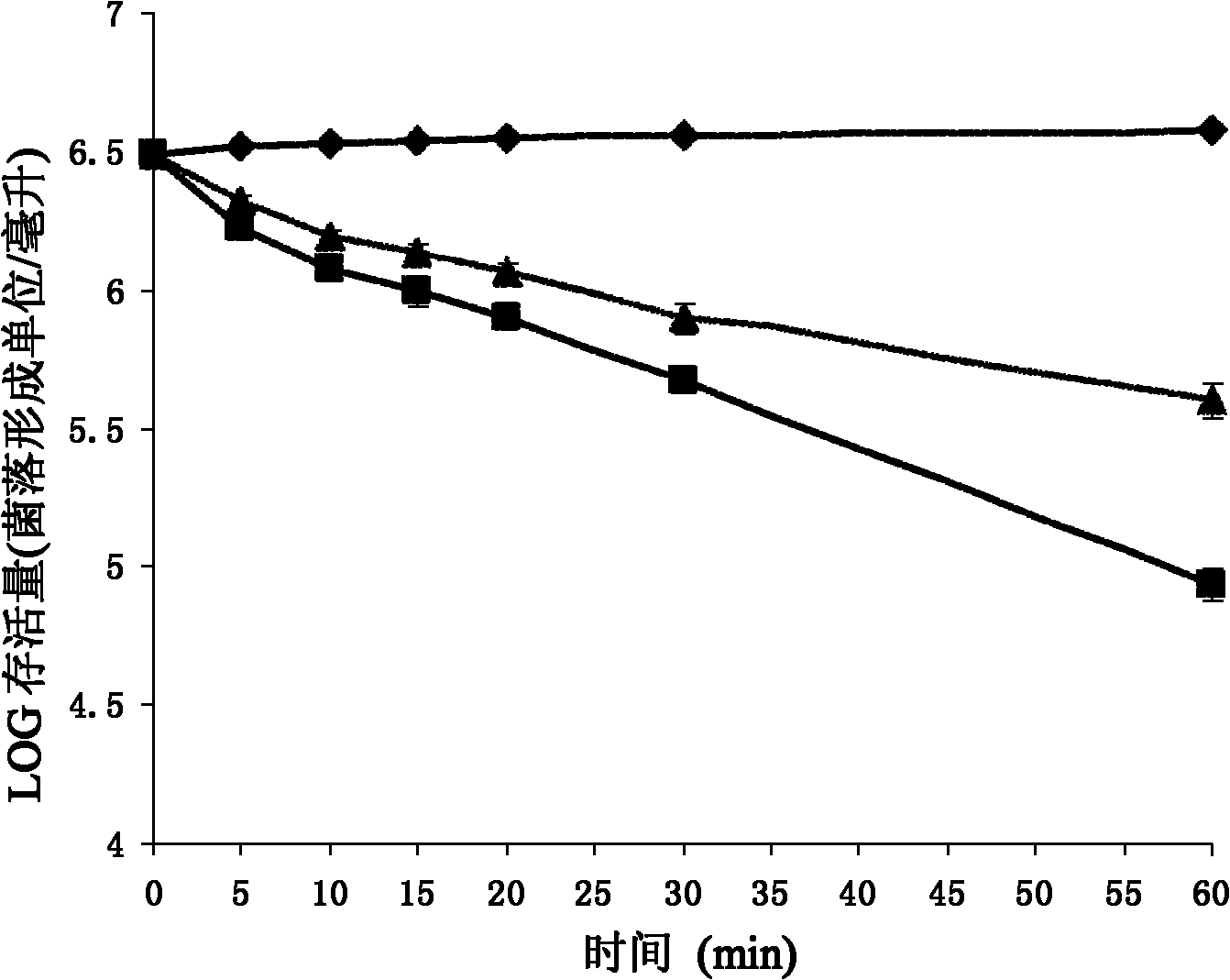
[0110] Dissolve chemically synthesized Pc-CATH1 in sterile ultrapure water at a concentration of 2 mg / ml; pick up newly activated microorganisms with an inoculation loop, and spread them evenly on a new LB agar plate; place a circle with a diameter of 0.5 cm Place a piece of sterile filter paper on the above-mentioned agar plate, and then drop 10 μl of Pc-CATH1 sample solution onto the piece of paper; put it in a constant temperature incubator at 37°C for 12-24 hours; CATH1-sensitive strains were recorded.
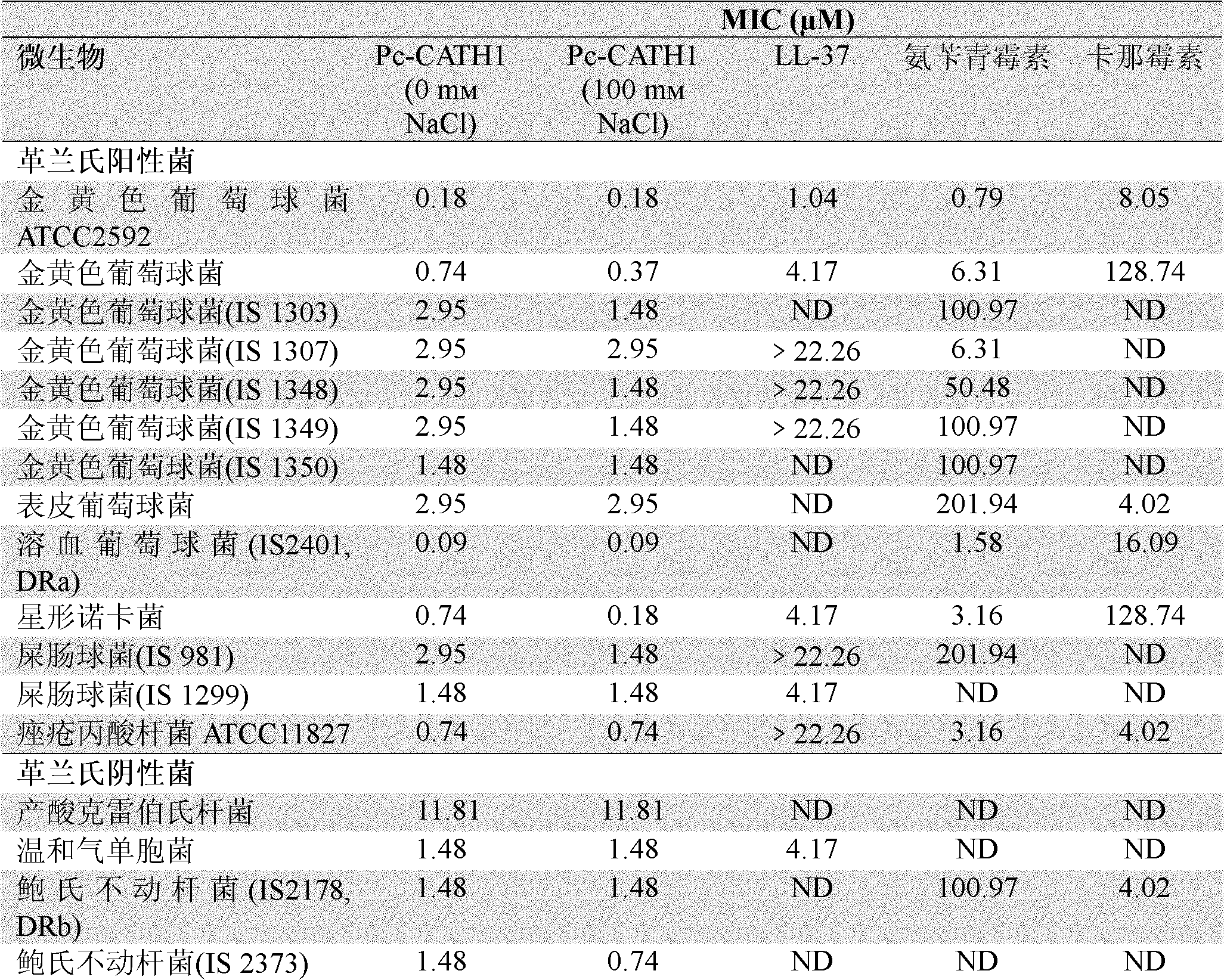
[0111] 2. Determination of Pc-CATH1 on the minimum inhibitory concentration (Minimum Inhibitory Concentration, MIC) of sensitive strains. In this experiment, human cathelicidin LL-37, traditional antibiotics ampicillin and kanamycin were used as positive controls, and sterile liquid LB was used as negative controls; the minimum inhibitory c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com