Tirofiban powder injection and its preparing method
A technology of rofiban powder and tirofiban, which is applied in the field of tirofiban powder injection, can solve the problems of poor physical stability of aqueous solution injection, difficult to maintain clarity, unstable pH value, etc. Stable properties and wide selection of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Preparation of tirofiban powder injection
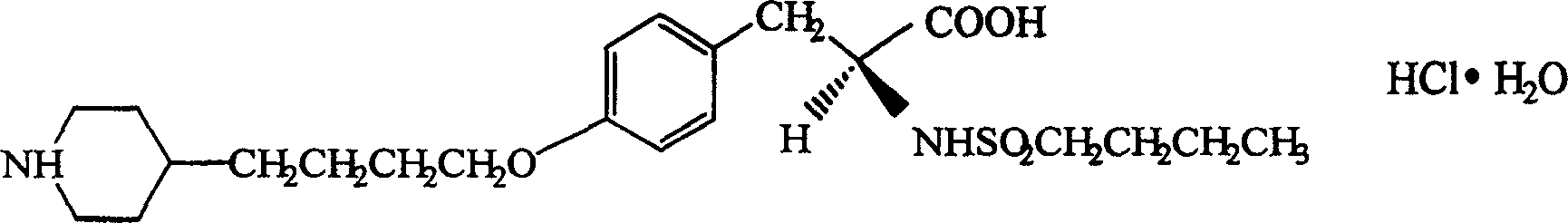
[0061] 1. According to the technical scheme disclosed in document Tetrahedron (1993) 49 (26) 5767-76, take tyrosine and butanesulfonyl chloride as raw materials to synthesize tirofiban.
[0062] 2. Prepare raw materials according to the table:
[0063] Ingredient Amount
[0064] Tirofiban Hydrochloride 5.6g
[0065] Sodium dihydrogen phosphate 8.0g
[0066] Disodium hydrogen phosphate 20.4g
[0067] Mannitol 50g
[0068] Water for injection 1000ml
[0069] 3. Preparation process:
[0070] Take by weighing disodium hydrogen phosphate, sodium dihydrogen phosphate, mannitol of prescribed quantity, add 800ml of water for injection to dissolve, add appropriate amount of active carbon for needles, stir, filter, add tirofiban hydrochloride of prescribed quantity, use 0.1mol / LHCl and 0.1mol / LNaOH adjust the pH value to 5.0-7.0 and add water for injection to 1000ml. Sterilize the medicinal solution according to the aseptic ...
Embodiment 2
[0072] Preparation of tirofiban powder injection
[0073] 1. According to the technical scheme disclosed in document Tetrahedron (1993) 49 (26) 5767-76, take tyrosine and butanesulfonyl chloride as raw materials to synthesize tirofiban.
[0074] 2. Prepare the required raw materials according to the table below:
[0075] Ingredient Amount
[0076] Tirofiban Hydrochloride 14.0g
[0077] Sodium dihydrogen phosphate 8.0g
[0078] Disodium hydrogen phosphate 20.4g
[0079] Mannitol 50g
[0080] Water for injection 1000ml
[0081] 3. Preparation process:
[0082] The preparation method is the same as in Example 1 except that the amount of tirofiban hydrochloride added is different.
Embodiment 3
[0084] Preparation of tirofiban powder injection
[0085] 1. According to the technical scheme disclosed in document Tetrahedron (1993) 49 (26) 5767-76, take tyrosine and butanesulfonyl chloride as raw materials to synthesize tirofiban.
[0086] 2. Prepare raw materials according to the table:
[0087] Ingredient Amount
[0088] Tirofiban Hydrochloride 5.6g
[0089] Sodium dihydrogen phosphate 8.0g
[0090] Disodium hydrogen phosphate 20.4g
[0091] Sorbitol 50g
[0092] Water for injection 1000ml
[0093] 3. Preparation process:
[0094] The preparation method is the same as in Example 1 except that the auxiliary materials added are different.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com