A Hydrophobic Prodrug-Based Gambogic Acid Nanoformulation with Improved Long Circulation
A nano-preparation, gambogic acid technology, applied in the field of medicine, can solve the problems of low drug loading efficiency, drug crystallization, and poor stability, and achieve the effects of improving drugability, reducing side effects, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of oleyl alcohol bromide
[0042]
[0043] Add 1 g of oleyl alcohol to the reaction vessel, add 20 ml of anhydrous dichloromethane to dissolve, then add 504.1 mg of phosphorus tribromide, stir at room temperature for 30 minutes, and separate 598.9 mg of white oily substance by silica gel column chromatography.
[0044] of the substance 1 The HNMR and MS data are as follows:
[0045] 1 HNMR (CDCl 3,300MHz)δ: 5.46-5.31(m,2H,9-H,10-H),3.43(t,J=6.9Hz,2H,1-H),2.10-1.89(m,4H,8-H, 11-H), 1.93–1.80 (m, 2H, 43-H), 1.50–1.25 (m, 22H), 0.89 (q, J=7.6, 6.9Hz, 3H).
[0046] MS(ESI) m / z: 663.45[2M+H] +
[0047] The chemical structural formula is as follows: C 18 H 35 Br.
Embodiment 2
[0048] Example 2: Preparation of gambogic acid-oleyl alcohol
[0049]
[0050] Add 1.03 g of gambogic acid to the reaction vessel, add N,N-dimethylformamide to dissolve, add 598.9 mg of oleyl bromide, then add 113.53 mg of potassium carbonate, stir at room temperature, overnight, and separate by silica gel column chromatography, namely The gambogic acid compound represented by formula (I), namely gambogic acid-oleyl alcohol, is obtained.
[0051] The 1HNMR and MS data of this material are as follows:
[0052] 1HNMR (CDCl3, 300MHz) δ: 12.84 (s, 1H, 6-OH), 7.53 (d, J=6.9Hz, 1H, 10-H), 6.66 (d, J=10.2Hz, 1H, 4-H) ,6.05–5.93(m,1H,27-H),5.46(d,1H,3-H),5.355(m,2H,50-H,51-H),5.11–4.98(m,2H,32- H, 37-H), 3.90–3.71 (m, 2H, 42-H), 3.47 (dd, J=6.9, 4.4Hz, 1H, 11-H), 3.31 (dd, J=14.6, 8.1Hz, 1H , 31-H), 3.21–3.10 (m, 1H, 31-H), 3.08–2.89 (m, 2H, 26-H), 2.51 (d, J=9.3Hz, 1H, 22-H), 2.30 ( dd, J=13.4, 4.8Hz, 1H, 21-H), 2.01 (q, J=6.9Hz, 6H, 36-H, 49-H, 52-H), 1.88–1.35 (m, 28H), 1.35...
Embodiment 3
[0055] Example 3: Preparation of gambogic acid-oleyl alcohol / vitamin E polyethylene glycol succinate nanoparticles (GA-OA / TPGS)
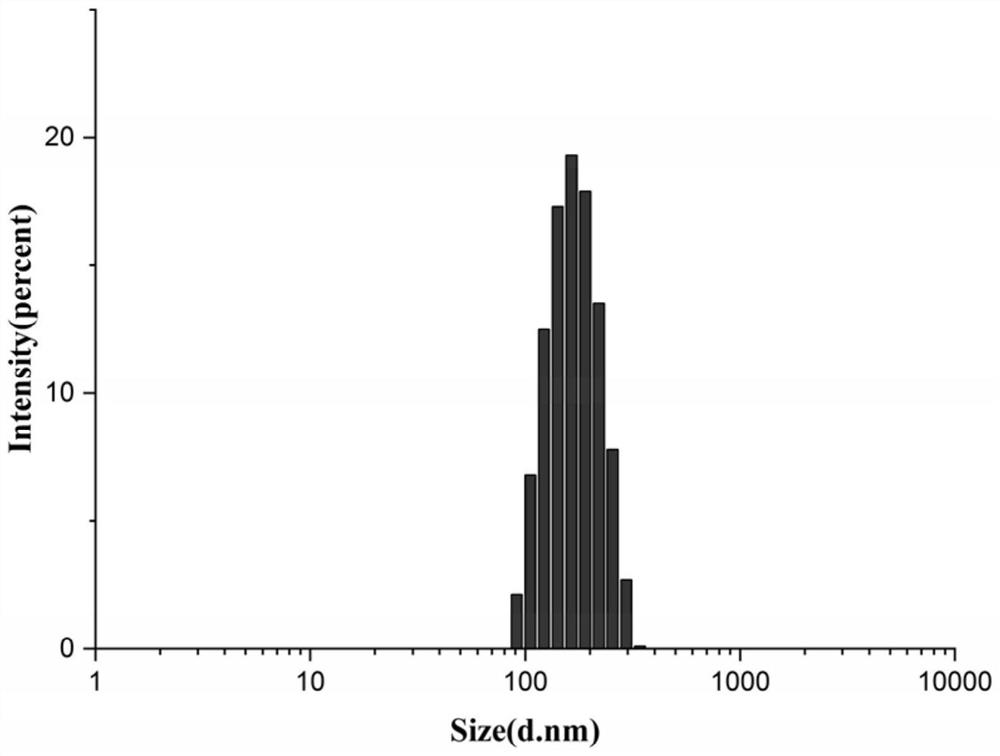
[0056] Weigh 7.15 mg of the gambogic acid-oleyl alcohol prodrug prepared in Example 2 and 1.0725 mg of vitamin E polyethylene glycol succinate (the molecular weight range of the polyethylene glycol contained is 1000-10000) and dissolve it in 800 μl DMSO. Slowly add the DMSO mixed solution to 10 ml of ultrapure water at 37°C, stir while adding, and let stand for 2 h, then dialyze for 2 to 5 days to remove DMSO to obtain gambogic acid-oleyl alcohol / vitamin E polyethylene glycol Succinate nanoparticles (GA-OA / TPGS) with appearance as figure 1 As shown, the particle size distribution is shown in figure 2 As shown, the average particle size was 155.23±3.34nm, the particle size distribution was narrow, the PDI was 0.078±0.027, and the drug loading and encapsulation efficiency were high, 56.82% and 65.60%, respectively. The gambogic acid-oleyl alcohol / v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com