Highly concentration water-soluble vitamin for intravenous injection and preparation and use
A technology of water-soluble vitamins and vitamins, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve problems such as inability to meet water-soluble vitamins, lack of supplementary needs, etc., to improve the effect of treatment or auxiliary treatment , reduce side effects, improve resistance and immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
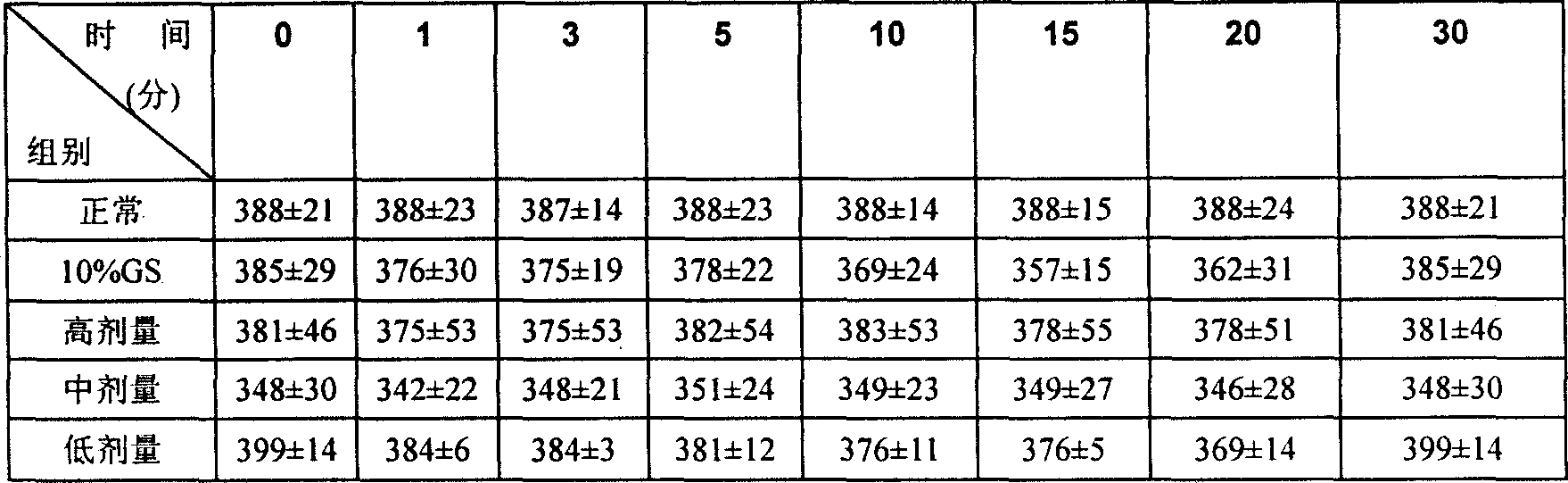
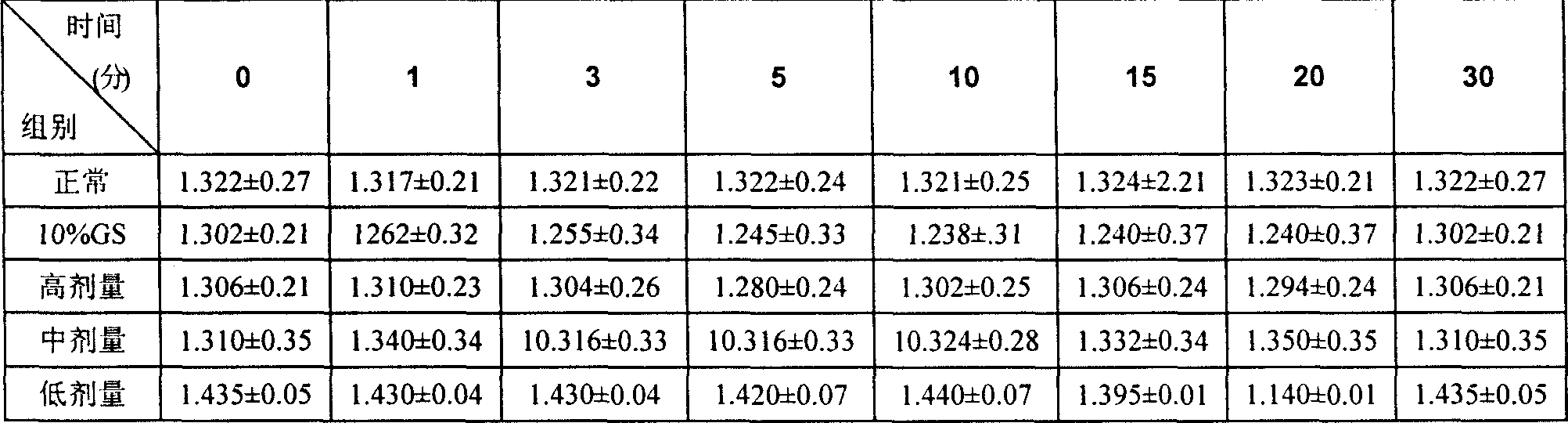
Image
Examples
Embodiment 1
[0015] Example 1: 1.86g thiamine nitrate, 2.94g riboflavin sodium phosphate, 24.0g nicotinamide, 2.94g pyridoxine hydrochloride, 9.9g sodium pantothenate, 60.0g vitamin C, 36mg biotin, 0.24g folic acid, 3.0 mg Vitamin B 12 , 0.15g methyl p-hydroxybenzoate, 0.15g disodium edetate, and 90g glycine were dissolved in water for injection, adjusted to 900ml with water for injection, filtered through a 0.22μg microporous membrane, subpackaged into 3ml bottles, and Freeze-dry in a freeze dryer, and press the cap to obtain the required high-concentration water-soluble vitamin freeze-dried powder injection for intravenous injection.
Embodiment 2
[0016] Example 2: 1.86g thiamine nitrate, 2.94g riboflavin sodium phosphate, 24.0g nicotinamide, 2.94g pyridoxine hydrochloride, 9.9g sodium pantothenate, 60.0g vitamin C, 36mg biotin, 0.24g folic acid, 3.0 mg Vitamin B 12 , Dissolve 90mg of disodium edetate in 300ml of water for injection, filter through a 0.22μg microporous membrane, pack into 300 bottles, freeze-dry in a lyophilizer, and press the cap to obtain the required high-concentration water-soluble solution for intravenous injection. Sexual vitamin freeze-dried powder injection.
Embodiment 3
[0017] Example 3: 1.42g thiamine nitrate, 3.60g riboflavin sodium phosphate, 27.3g nicotinamide, 2.28g pyridoxine hydrochloride, 7.63g sodium pantothenate, 53.0g vitamin C, 42mg biotin, 0.20g folic acid, 2.4 mg Vitamin B 12 , Dissolve 90mg of disodium edetate in 300ml of water for injection, filter through a 0.22μg microporous membrane, pack into 300 bottles, freeze-dry in a lyophilizer, and press the cap to obtain the required high-concentration water-soluble solution for intravenous injection. Sexual vitamin freeze-dried powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com