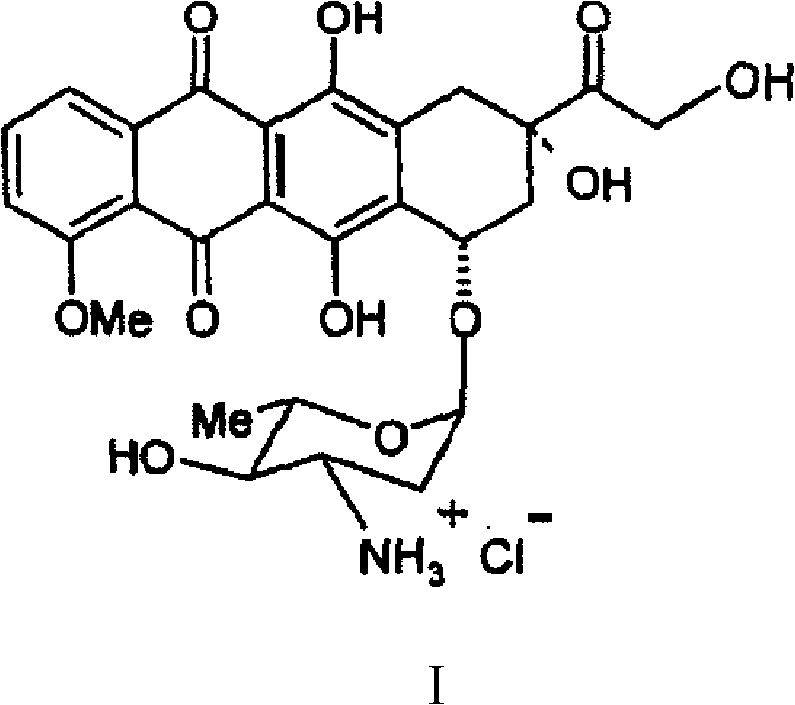
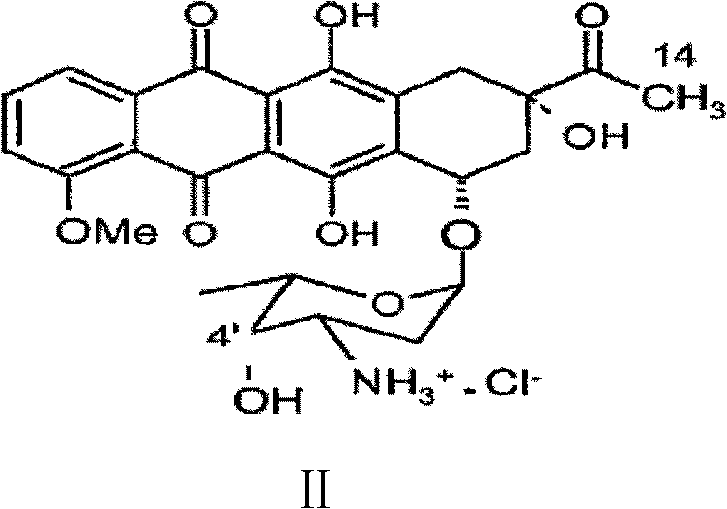
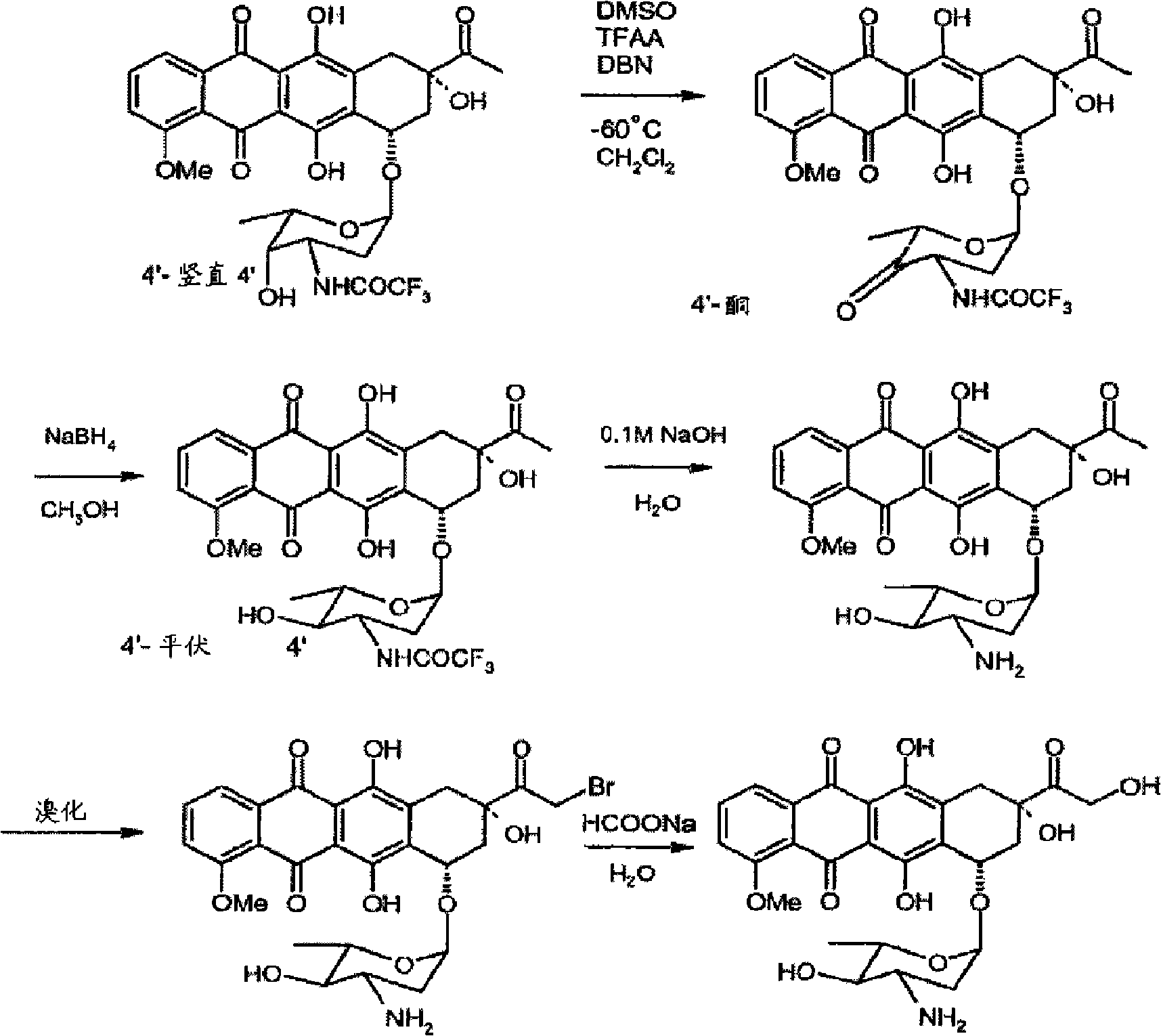
Preparation method for intermediate of epirubicin hydrochloride
A technology of epirubicin hydrochloride and intermediates, which is applied in the field of medicine, can solve the problems of poor reduction selectivity and side reactions of sodium borohydride, achieve mild reaction conditions, prevent by-products, and reduce side reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1N-trifluoroacetyl-daunorubicin
[0033] Add 80L of dichloromethane and 750g of daunorubicin hydrochloride to the reaction kettle, stir under the protection of argon, cool down to 0°C, add 1.3L of trifluoroacetic anhydride dropwise, stir for 1 hour, and add 20L of methanol to the reaction solution Use sodium bicarbonate aqueous solution to adjust pH=7.0~8.0, stir at 20±5°C for 3~5 hours, TLC detection and control reaction, use chloroform / isopropanol (volume ratio=96 / 4) as developer, use raw material Until the reaction is complete or almost disappears, intermediate I Rf ≈ 0.3, daunorubicin Rf ≈ 0, separate the organic phase and concentrate it to about 20L under reduced pressure, add 20L ethyl acetate, and concentrate under reduced pressure to 5~ 8 L, directly added to 65 L of n-hexane for crystallization under stirring, and suction-filtered and dried to obtain 739 g of N-trifluoroacetyl-daunorubicin as a solid, with a yield of 95%.
Embodiment 2
[0034] The preparation of embodiment 2N-trifluoroacetyl-daunorubicin
[0035] Add 60L of dichloromethane and 750g of daunorubicin hydrochloride to the reaction kettle, stir under the protection of argon, cool down to 5°C, add 1.1-1.3L of trifluoroacetic anhydride dropwise, stir for 1 hour, and add 20L of After methanol, use sodium bicarbonate aqueous solution to adjust pH=7.0~8.0, stir at 20±5°C for 3~5 hours, TLC detection and control reaction, using chloroform / isopropanol (volume ratio=96 / 4) as developing solvent, Until the raw material reacts completely or almost disappears. Intermediate I Rf≈0.3, daunorubicin Rf≈0, separate the organic phase and concentrate it under reduced pressure to about 15L, add 20L ethyl acetate, concentrate under reduced pressure to 5-8L at a water bath temperature of 50°C, and directly add to 65L of n-hexane was crystallized, filtered and dried to obtain 735g of N-trifluoroacetyl-daunorubicin as a solid, with a yield of 93%.
Embodiment 34
[0036]The preparation of embodiment 34'-keto-N-trifluoroacetyl-daunorubicin
[0037] Put 30L of dichloromethane and 0.9L of dimethyl sulfoxide into the reaction kettle, lower the temperature to -70°C under the protection of argon, slowly add 532.9g of phosphorus pentoxide, keep stirring for 20 to 40 minutes, and dissolve 780g of N-trifluoroacetyl - Dissolve daunorubicin in 15L of dichloromethane, slowly add to the above reaction solution, dropwise for 60-70 minutes, stir for 2 hours, then slowly drop into the system 0.65L of triethylamine, stir at -30°C React for 1 hour, directly add a mixed solution of 0.6L glacial acetic acid and 4L dichloromethane, stir for more than about 10 minutes, TLC to detect the reaction progress, using chloroform / isopropanol (volume ratio=96 / 4) as the developer, Until intermediate I disappears or almost disappears, intermediate I Rf ≈ 0.3, intermediate II Rf ≈ 0.8, the temperature of the reaction solution is raised to 0-10°C, and the organic phase i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com