Pseudomonas engineering bacteria for producing epirubicin
A technology of epirubicin and Pseudomonas putida, applied in the field of genetic engineering, can solve problems such as strict requirements, restrictions on effective development and application of epirubicin, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
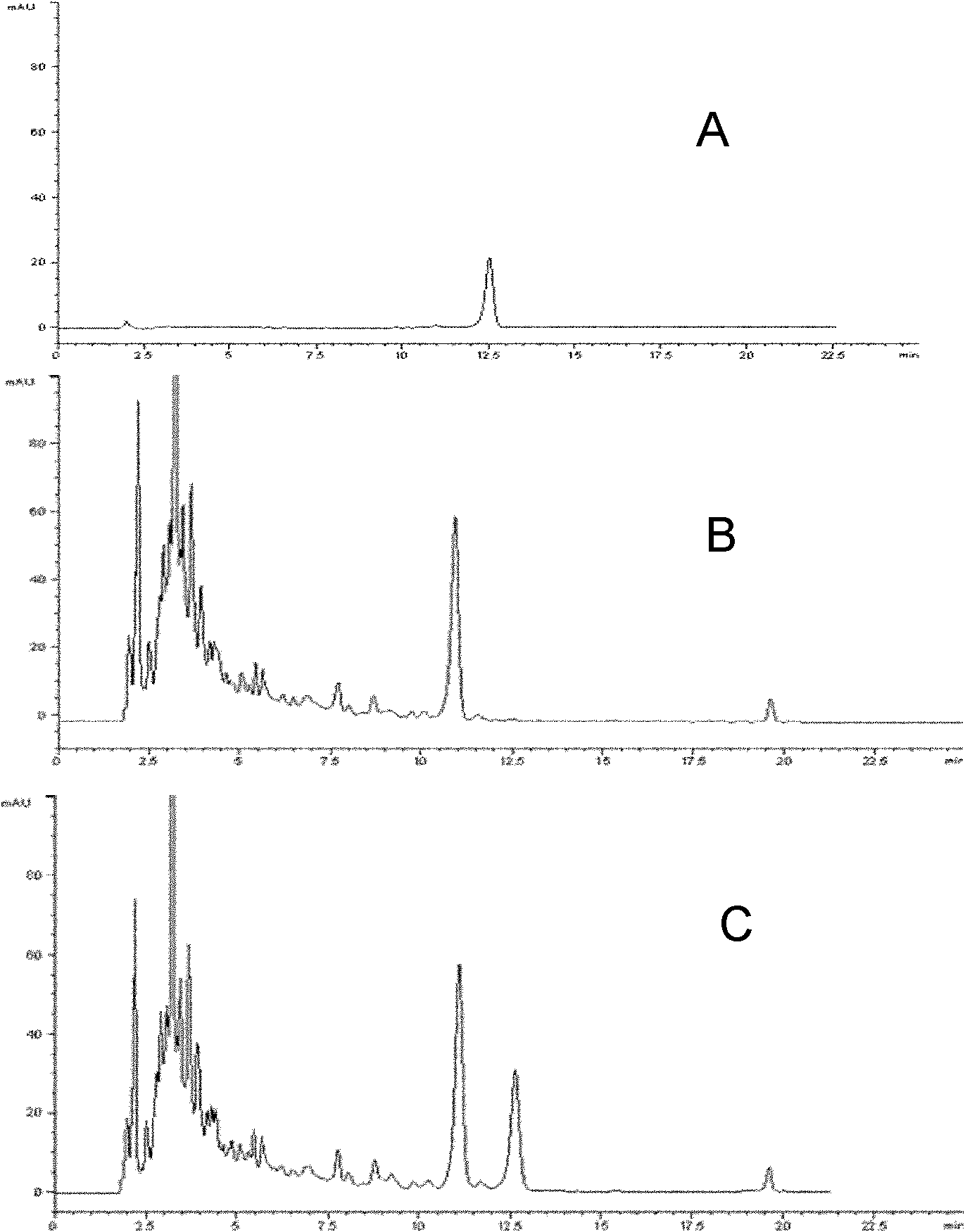
Examples
Embodiment 1
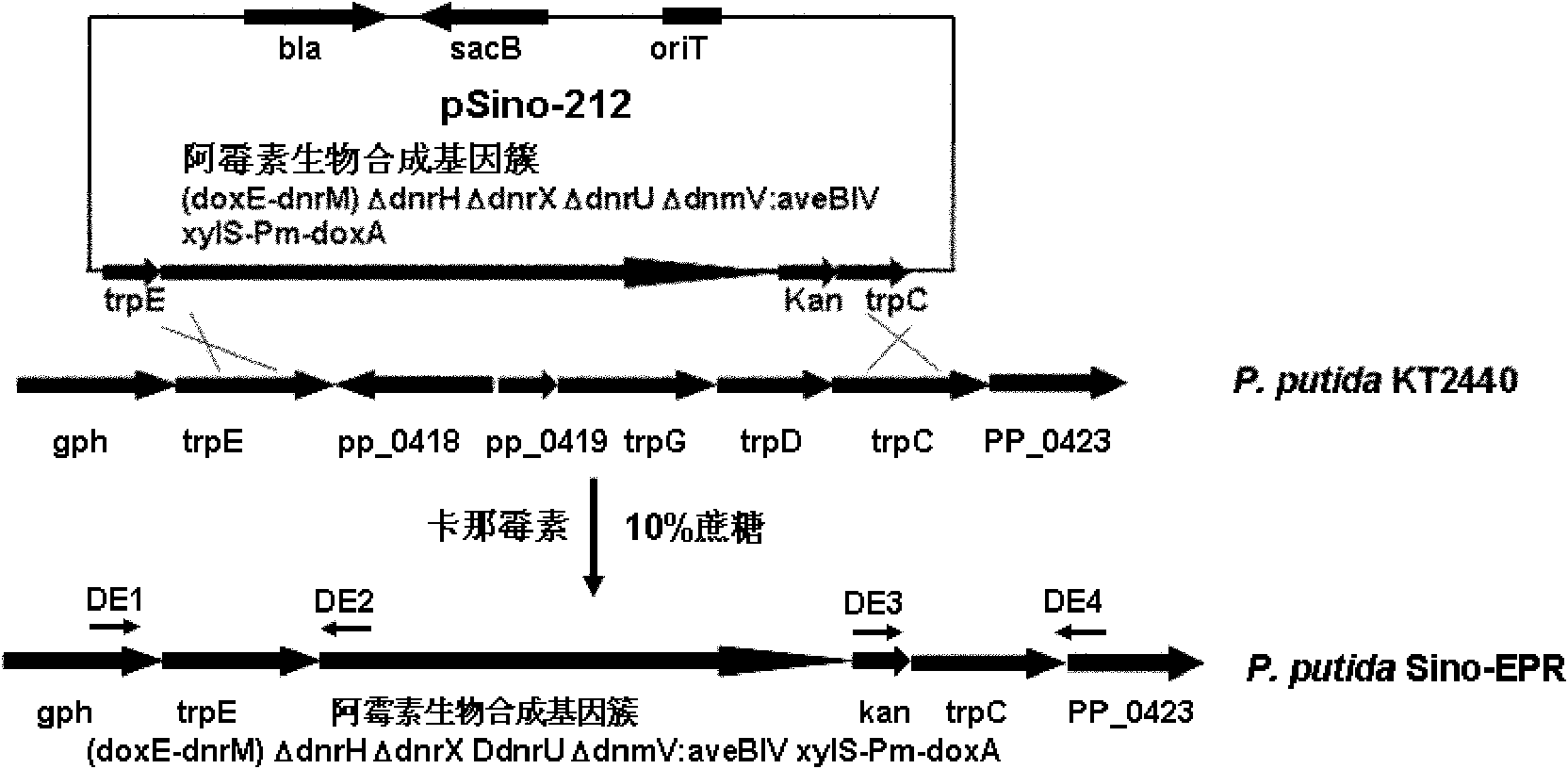
[0040] Example 1 Construction of doxorubicin biosynthesis gene cluster cloning vector
[0041] In order to construct the expression vector of doxorubicin biosynthetic gene cluster, which was then integrated into the P.putida KT2440 genome for expression, the transformation of low-copy plasmids and the cloning of homologous fragments of the P.putida KT2440 genome were carried out successively. Cloning of homology arms of synthetic gene clusters and cloning of positive and negative selection markers.
[0042] 1. Preparation and DNA transformation of competent Escherichia coli DH10B
[0043] Pick a single colony of Escherichia coli DH10B freshly streaked from the plate, put it into 2ml LB liquid medium and shake overnight at 37°C, transfer it to 50ml LB at 1 / 50 volume, and shake it until the cell OD 600 About 0.6. Pour the bacterial solution into a pre-cooled centrifuge tube, place in an ice bath for 10 minutes, centrifuge at 5000 rpm for 5 minutes at 4°C, and discard the super...
Embodiment 2
[0076] Cloning and modification of embodiment 2 doxorubicin biosynthesis gene cluster
[0077] The invention utilizes the recombination engineering method to clone and modify the biosynthetic gene cluster of doxorubicin.
[0078] 1. Construction of a plasmid expressing the recombinase gene
[0079] Due to the many resistance markers involved in the cloning gene cluster experiment, we first constructed a recombinase expression plasmid with chloramphenicol resistance.
[0080] Design primers RC1: 5'-AAACATGGATATTAATACTGAAACTG-3', RC2: 5'-AAAAAGCTTGTCATCGCCATTGCTCCCCAAATAC-3', using lambda DNA as a template, PCR amplified to obtain a 1.9kb recombinase gene (ie gam, bet and exo gene) fragment, After digestion with NcoI and HindIII, it was cloned into the same site of pBAD322C, and the resulting plasmid was named pBADRed.
[0081] Since pBADRed and pDHA5 contain the same replicon, in order to avoid plasmid incompatibility, pBADRed was digested with NsiI and PstI, and the 3.2kb ar...
Embodiment 3
[0098] Example 3 Gene knockout of dnrH, dnrX and dnrU in the doxorubicin biosynthetic gene cluster
[0099] The genes dnrH, dnrX, and dnrU are negative regulatory genes in the doxorubicin biosynthetic gene cluster, DnrH and DnrX catalyze the conversion of doxorubicin and daunomycin to baumycin-like glycosides, and DnrU catalyzes the conversion of daunomycin Converted into 13(R)-dihydroxydaunomycin, which are all by-products of doxorubicin metabolism, blocking their formation is beneficial to the biosynthetic precursor small molecule metabolic flow to the target product doxorubicin, thereby increasing The yield of doxorubicin while eliminating by-products facilitates the isolation and purification of the target product.
[0100] 1. Knockout of dnrH gene
[0101] The gene knockout of dnrH was carried out by recombination engineering method. Firstly, dnrH gene was replaced by positive selection and negative selection gene cassette rpsL-tet through homologous recombination, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com