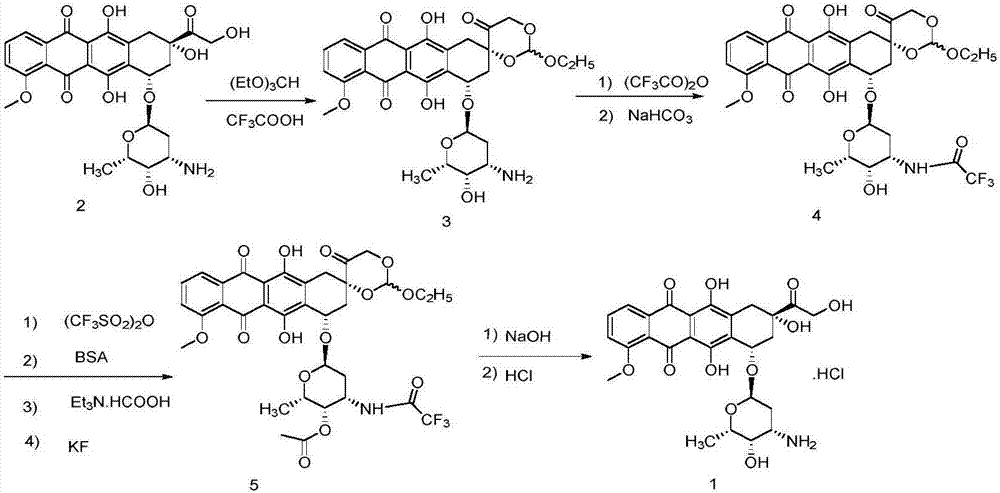
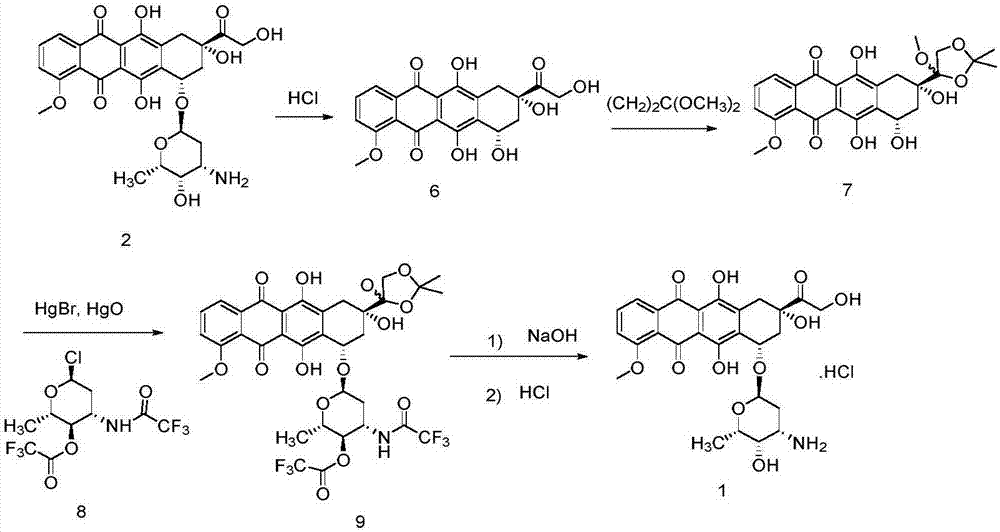
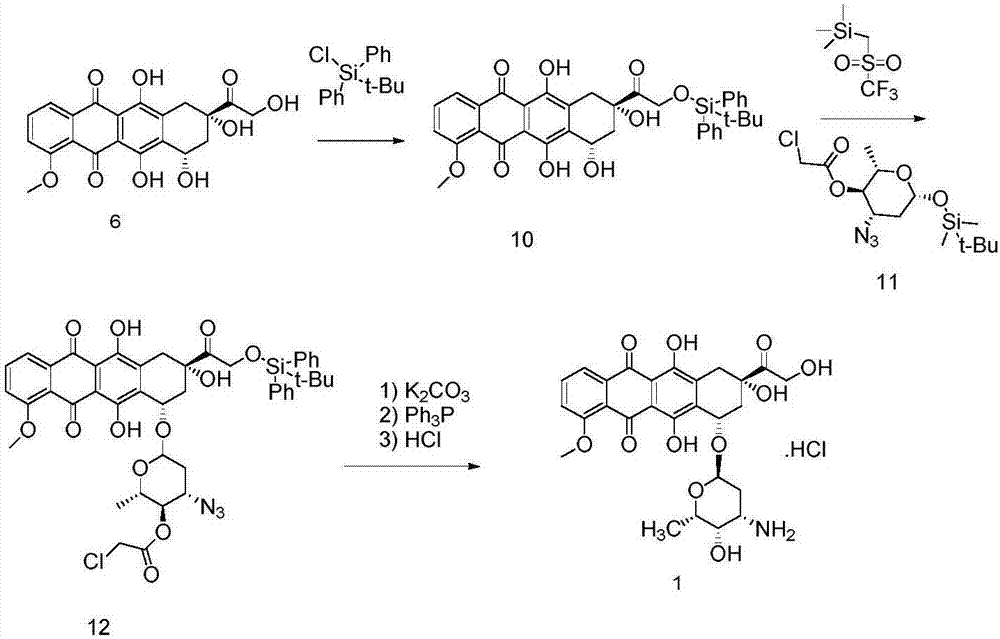
Method for preparing epirubicin hydrochloride and intermediate compound
A technology for epirubicin hydrochloride and intermediates, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of long reaction route, high cost, and heavy metal pollution, and reduce the consumption of raw materials , reduce the cost of response, and improve the effect of core competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1. Preparation of intermediate compounds
[0057] Under the protection of nitrogen, install a stirrer and a thermometer in a 500mL three-necked flask, add 300mL of dichloromethane and 54.3g (100mmol) of doxorubicin as a raw material, and cool to -20°C. At this temperature, add 16.8g (60mmol) of 2-iodobenzoic acid IBX and 39.0g (50mmol) of dimethyl sulfoxide DMSO in batches, continue stirring for 3 hours, rise to 0°C and continue stirring for 0.5 hours, and slowly rise to room temperature . Add 300 mL of dichloromethane to dilute, filter, and wash the organic phase with 0.1M dilute hydrochloric acid solution, saturated sodium bicarbonate solution, and saturated brine, respectively. Dry over anhydrous magnesium sulfate, and concentrate the organic phase to obtain an oily liquid. Add 300 mL of n-hexane to this liquid, crystallize under slow stirring at -5°C, and filter to obtain an orange powdery solid intermediate. It is determined that the yield of the intermediate re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com