High-safety ropivacaine hydrochloride injection and preparation method thereof
A high-safety technology of ropivacaine hydrochloride, applied in the field of medicine, can solve the problems of adding a large amount of desiccation protective agent, freeze-dried powder injection, unqualified impurities in clarity, poor stability, etc., to ensure physical stability and biological stability Sex, reduce the chance of bacterial contamination, good adsorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] prescription:
[0033] Ropivacaine hydrochloride 50g,
[0035] Sodium hydroxide or hydrochloric acid qs, and
[0036] Add water for injection to 10000ml;
[0037] The formula is made into 1000 injections, and the pH of the injection is 5.0;
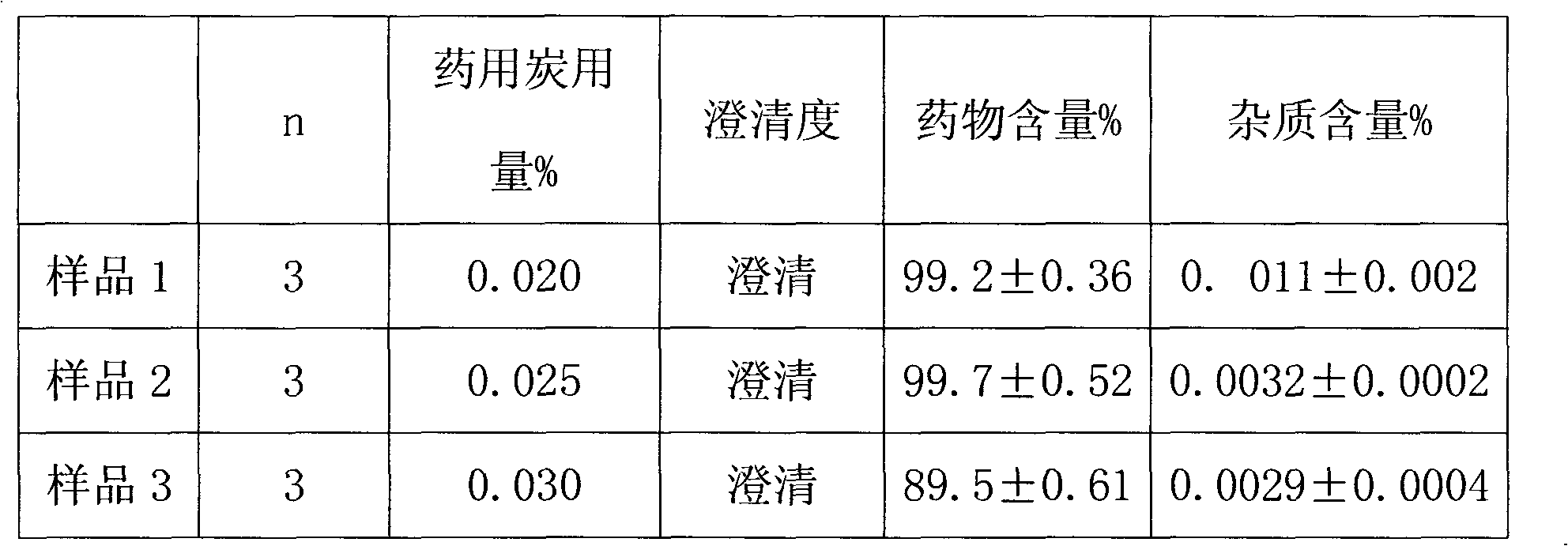
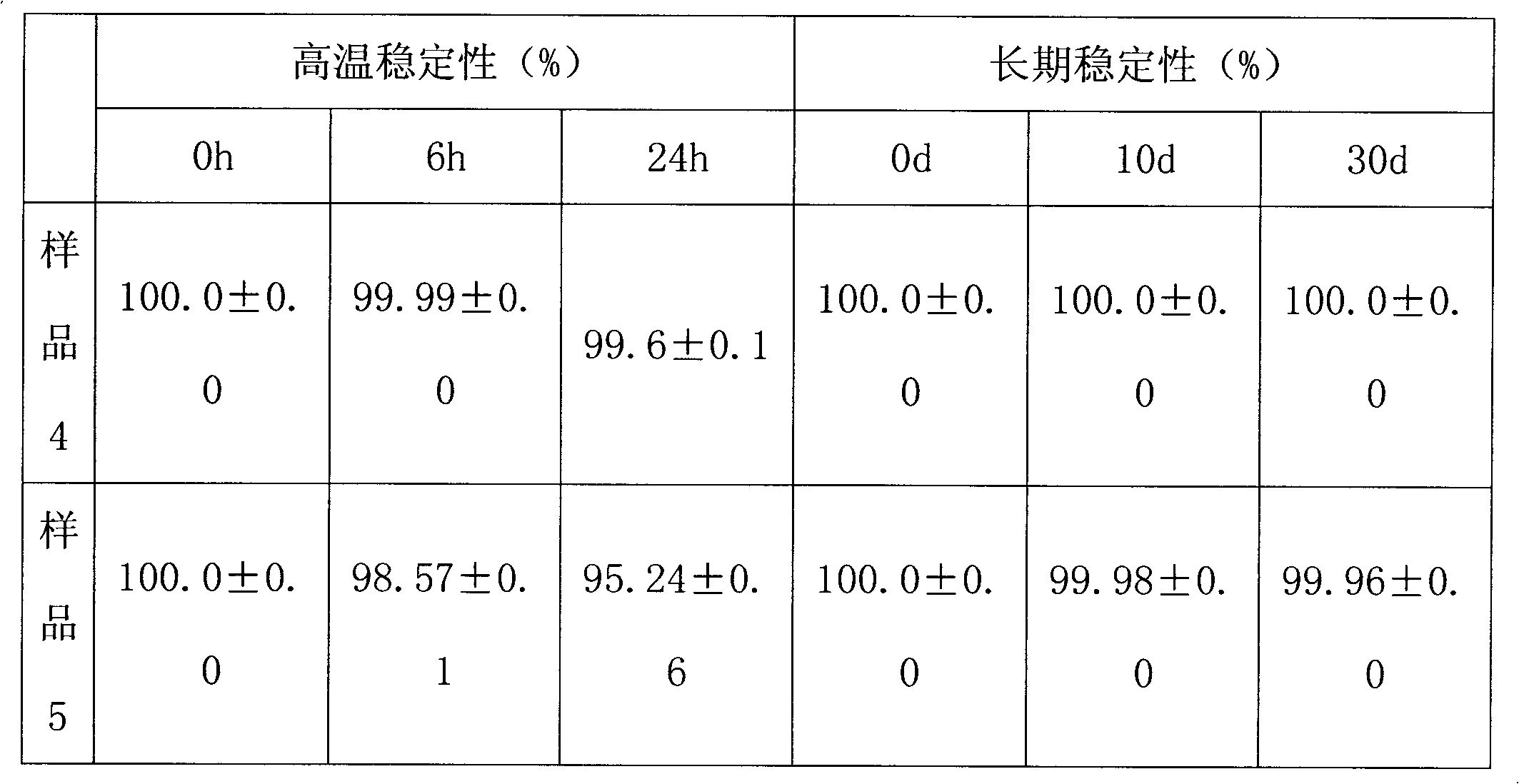
[0038] The preparation method is as follows: stirring and dissolving ropivacaine hydrochloride and sodium chloride in 1 / 3 volume of water for injection of the whole amount, adding an appropriate amount of sodium hydroxide or hydrochloric acid, and adjusting the pH value to 5.0; The liquid medicine is circulated and filtered and adsorbed in a plate and frame filter containing 0.01% activated carbon for 10 minutes to perform coarse filtration, and the content and pH value of the intermediate product are measured. After passing the test, filter with a 0.22 μm microporous membrane; To the full amount, stirred for 10 minutes and left to stand for 0.5 hours, then canned in a high-temperature-resistant pl...
Embodiment 2
[0040] prescription:
[0041] Ropivacaine Hydrochloride 75g,
[0042] Sodium chloride 75g,
[0043] Sodium hydroxide or hydrochloric acid qs, and
[0044] Add water for injection to 10000ml;
[0045] The formula is made into 1000 injections, and the pH of the injection is 5.0;
[0046] The preparation method is as follows: stirring and dissolving ropivacaine hydrochloride and sodium chloride in 4 / 5 volume of water for injection of the whole amount, adding appropriate amount of sodium hydroxide or hydrochloric acid, and adjusting the pH value to 5.0; The liquid medicine is circulated, filtered and adsorbed for 60 minutes in a plate-and-frame filter containing 0.5% activated carbon to carry out coarse filtration, and the content and pH value of the intermediate product are measured. After passing the test, filter it with a 0.22 μm microporous membrane; To the full amount, stir for 10 minutes and let it stand for 2 hours, then can it in a high-temperature-resistant plastic co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com