Hypocrellin liposome preparation and preparation method thereof
A technology of liposome preparation and hypocretin, which is applied in the direction of liposome delivery, pharmaceutical formulation, medical preparation of non-active ingredients, etc., can solve the problems affecting the monodisperse shape stability of liposome solid powder, The quality of batch finished products is not easy to control, and the bilayer phospholipid film is damaged, etc., to achieve the effect of good monodispersity, increased yield, and stable shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
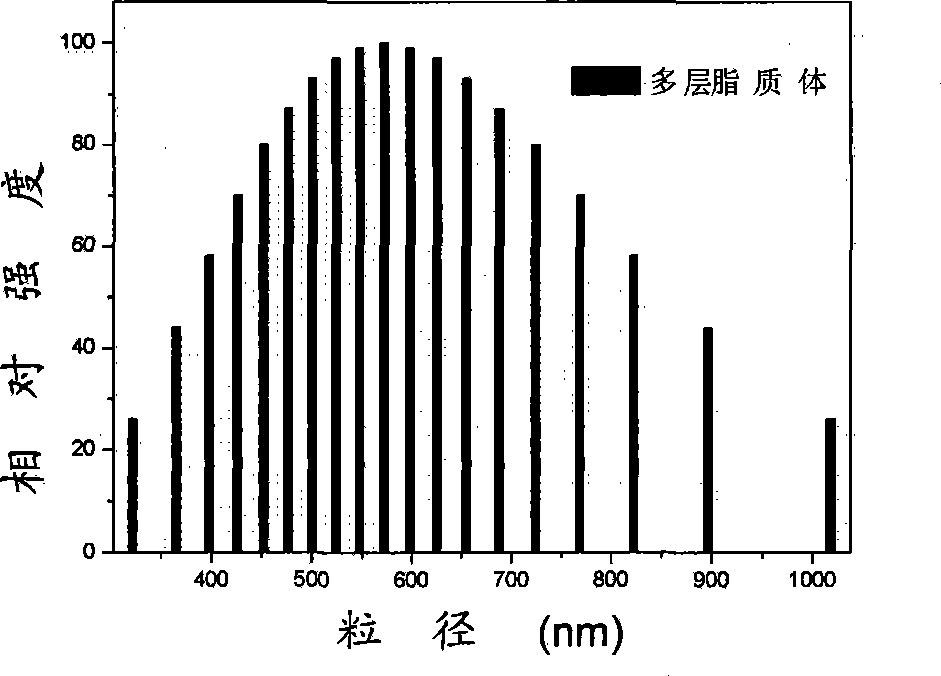
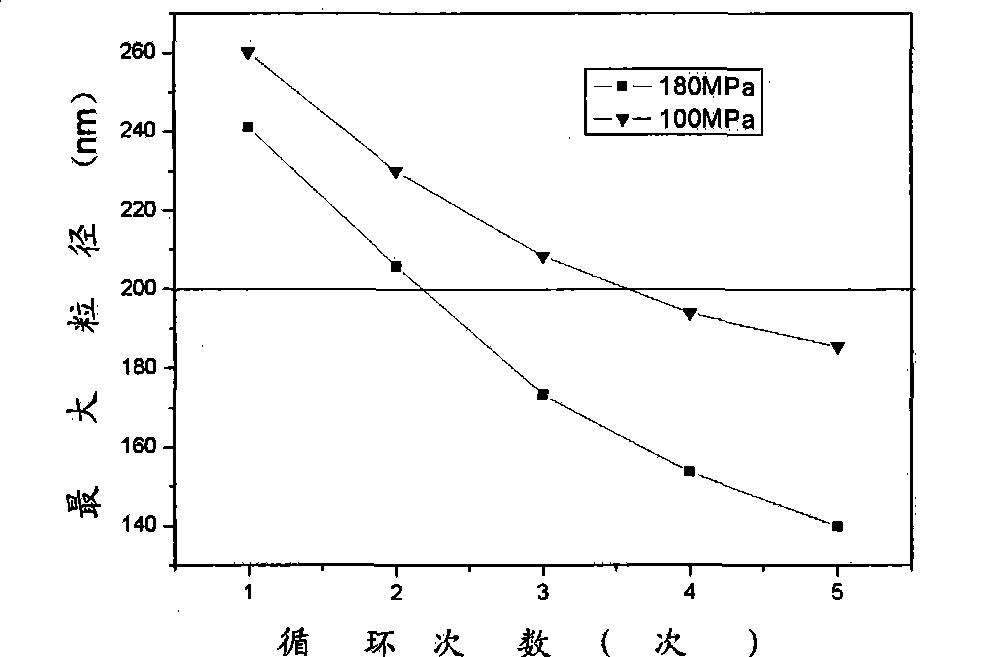
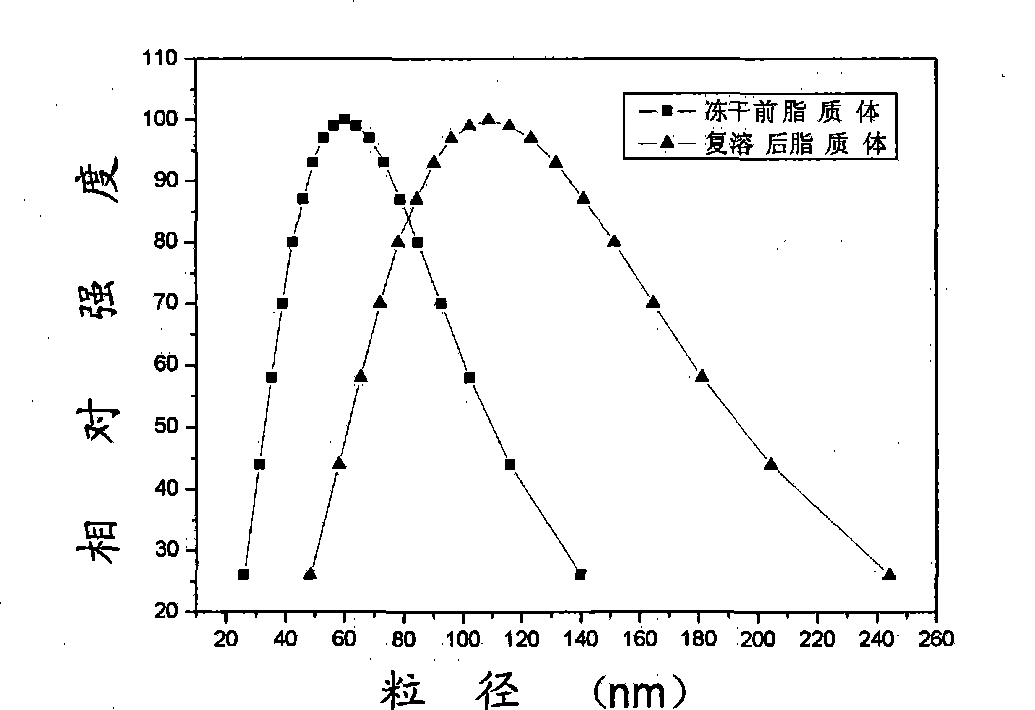
[0031] 100mg hypocrellin A, 4g lecithin, 240mg cholesterol and 97.7mL water were emulsified to make multilamellar liposomes with a particle size below 1000nm. Pour it into a high-pressure homogenizer at 10MPa for initial homogenization, then pressurize to 180MPa for high-pressure homogenization, and keep the system temperature at 15 0C, cycled 5 times to obtain single-lamellar liposomes with a particle size distribution of 46-140 nm. Add 4g of sucrose, shake and dissolve, sterilize by ultrafiltration through a filter membrane with a pore size of 220nm, put it in a -80°C refrigerator for rapid pre-freezing, and freeze-dry the obtained freeze-dried liposome powder. The content is 1.20%.
[0032] Among them, the particle size distribution of multilamellar liposomes is shown in figure 1 Shown; the particle size distribution of liposomes before freeze-drying and liposomes after reconstitution image 3 Shown; The transmission electron micrograph of liposome diluted to 0.05mg / ml b...
Embodiment 2
[0035] 200mg Hypocretin A, 6g hydrogenated soybean lecithin, 480mg cholesterol and 195.4mL water were emulsified to make multilamellar liposomes with a particle size below 1000nm. Pour it into a high-pressure homogenizer at 15MPa for initial homogenization, then pressurize to 200MPa for high-pressure homogenization, and keep the system temperature at 10 0 C, cycled twice to obtain single-lamellar liposomes with a particle size distribution of 40-129 nm. Add 8g of mannose, shake and dissolve, sterilize by ultrafiltration through a 220nm filter membrane, put it in a -60°C refrigerator for rapid pre-freezing, freeze-dry the obtained freeze-dried liposome powder and store it in an inert gas seal. The drug content is 1.36%.
Embodiment 3
[0037] 500mg Hypocretin A, 20g hydrogenated lecithin, 1.2g cholesterol and 488.5mL water were emulsified to make multilamellar liposomes with a particle size below 1000nm. Pour it into a high-pressure homogenizer at 20MPa for initial homogenization, then pressurize to 130MPa for high-pressure homogenization, and keep the system temperature at 5 0 C, cycled 7 times to obtain unilamellar liposomes with a particle size distribution of 49-162 nm. Add 10g of sucrose, shake and dissolve, sterilize by ultrafiltration through a filter membrane with a pore size of 220nm, put it in a -70°C refrigerator for rapid pre-freezing, and freeze-dry the obtained freeze-dried liposome powder. The content is 1.58%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com