Drug balloon
A balloon and drug technology, applied in the field of medical devices, can solve the problems of insufficient smooth muscle cell inhibition, decreased lumen patency rate, drug toxicity, etc., to achieve the goal of inhibiting excessive proliferation of smooth muscle cells, improving patency rate, and high drug content Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment ( 1
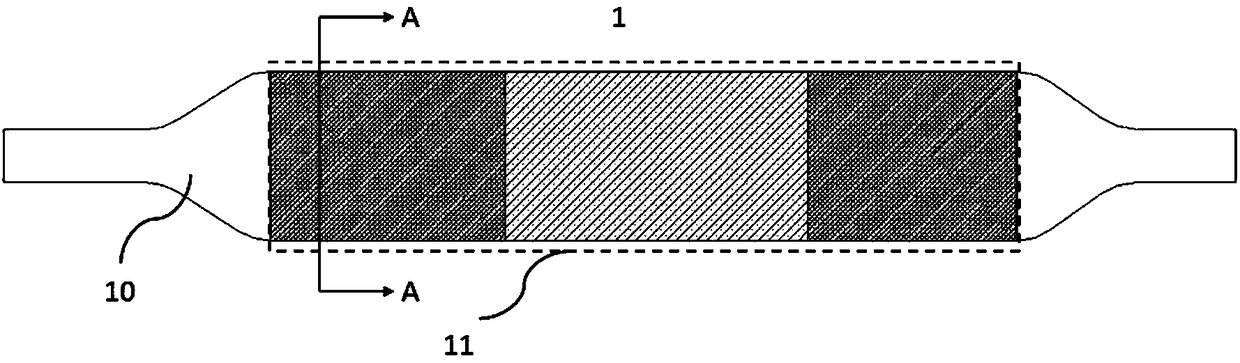
[0035] Please refer to Figures 1(a), 2(a) and image 3 . Figure 1(a) is a schematic structural view of a drug balloon 1 in Example 1 of the present invention; Figure 2(a) is a schematic structural view of a drug coating 11; image 3 It is a structural schematic diagram of the cross section of the drug balloon 1 . In this implementation, the drug balloon 1 includes a balloon 10 and a drug coating 11 .
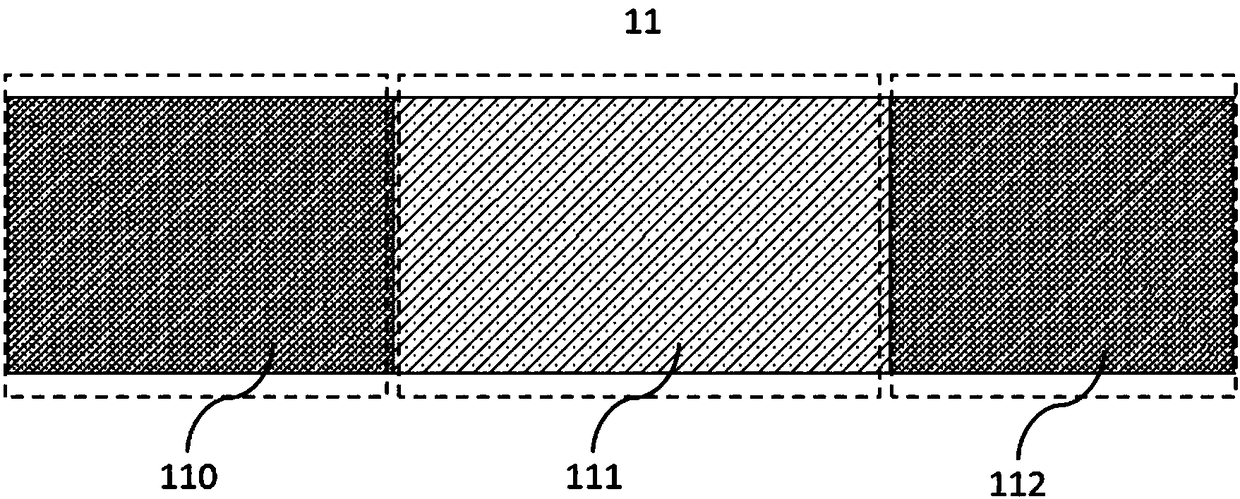
[0036]Wherein, the drug coating 11 is located on the surface of the balloon and includes a drug 113 and an excipient 114 . The drug coating 11 includes a proximal coating 110, a middle coating 111 and a distal coating 112, and the proximal coating 110, the middle coating 111 and the distal coating 112 are sequentially arranged and connected, distributed on the balloon 10 The outer surface. The drug loading density in the proximal coating 110 is greater than that in the middle coating 111 , and the drug loading density in the distal coating 112 is greater than that in the middl...
Embodiment ( 2
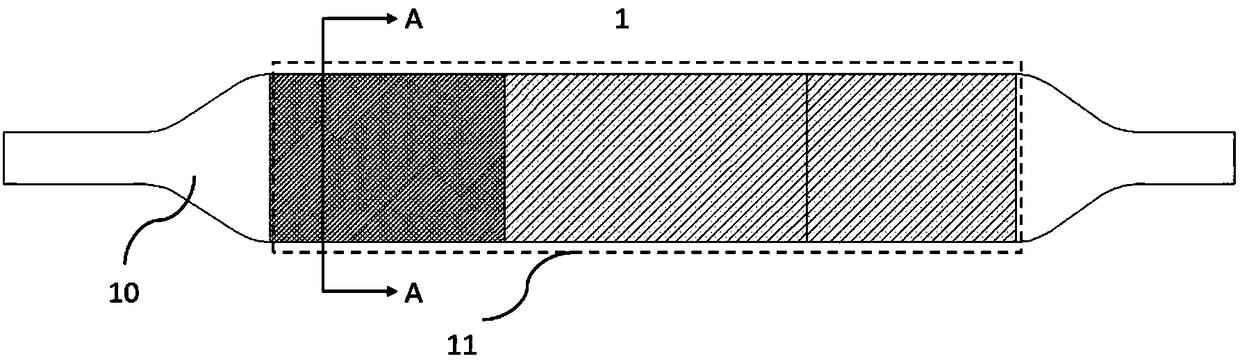
[0047] Please refer to Figures 1(b), 2(b) and image 3 . Figure 1(b) is a schematic structural view of the drug balloon 1 in Example 2 of the present invention; Figure 2(b) is a schematic structural view of the drug coating 11; image 3 It is a structural schematic diagram of the cross section of the drug balloon 1 . In this embodiment, the drug balloon 1 includes a balloon 10 and a drug coating 11 .
[0048] Wherein, the drug coating 11 is located on the surface of the balloon, including a proximal coating 110, a middle coating 111 and a distal coating 112, and the proximal coating 110, the middle coating 111 and the distal coating 112 are sequentially arranged and connected . Drug coating 11 includes drug 113 and excipient 114 . The drug loading density in the proximal coating 110 is greater than that in the middle coating 111 , and the drug loading density in the distal coating 112 is equal to the drug loading density in the middle coating 111 . In other embodiments, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com