Heterocyclic carboxylic acid-modified thymopentins and their preparation method, anti-tumor effect and use
A technology of heterocyclic carboxylic acid and thymus, applied to inhibit the activity of tumor body weight gain in S180 tumor mice, the application in the preparation of anti-tumor drugs, in vitro anti-tumor cell proliferation activity, thymopentin field, can solve the problem of insufficient curative effect Ideal, high toxicity and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
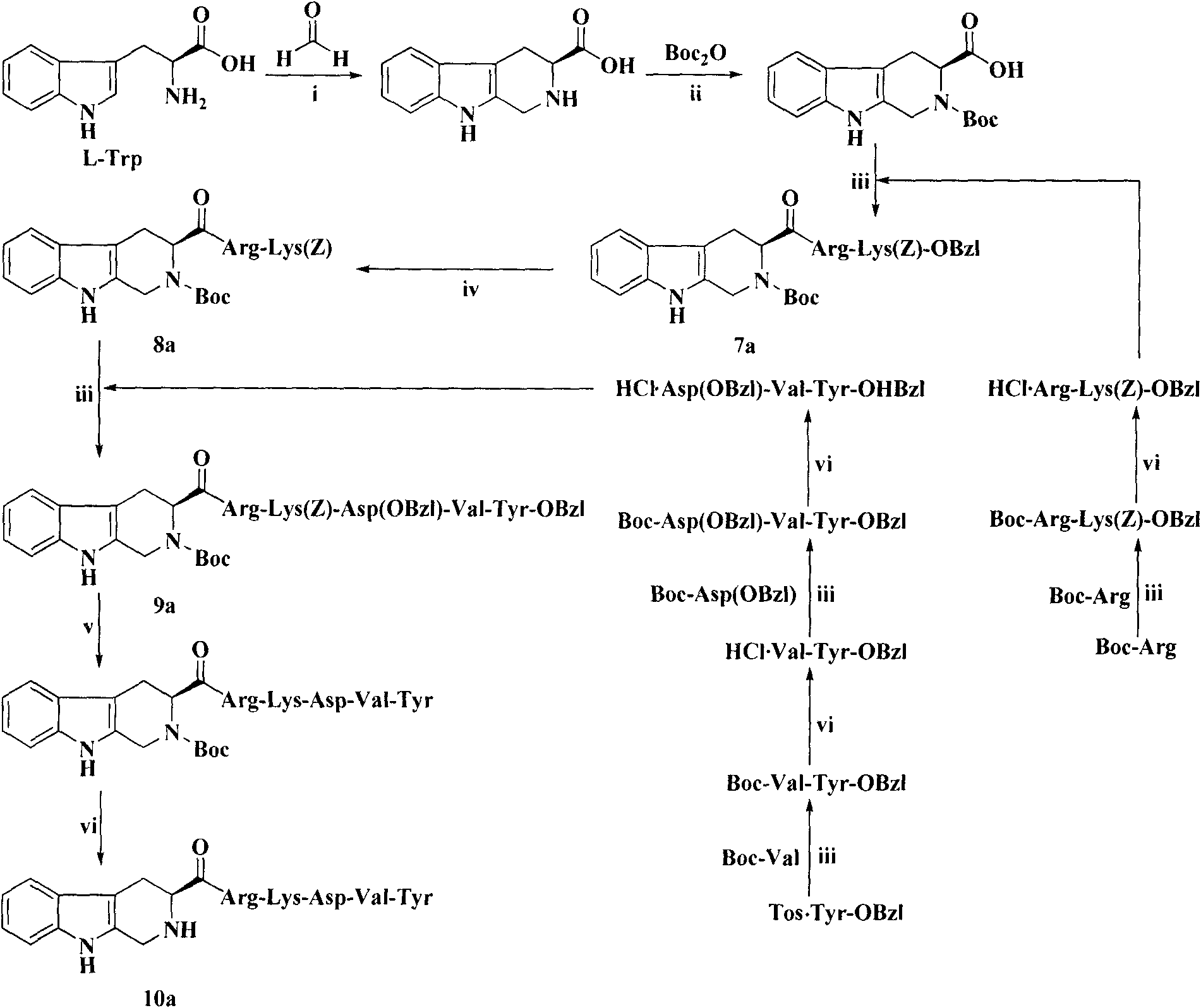
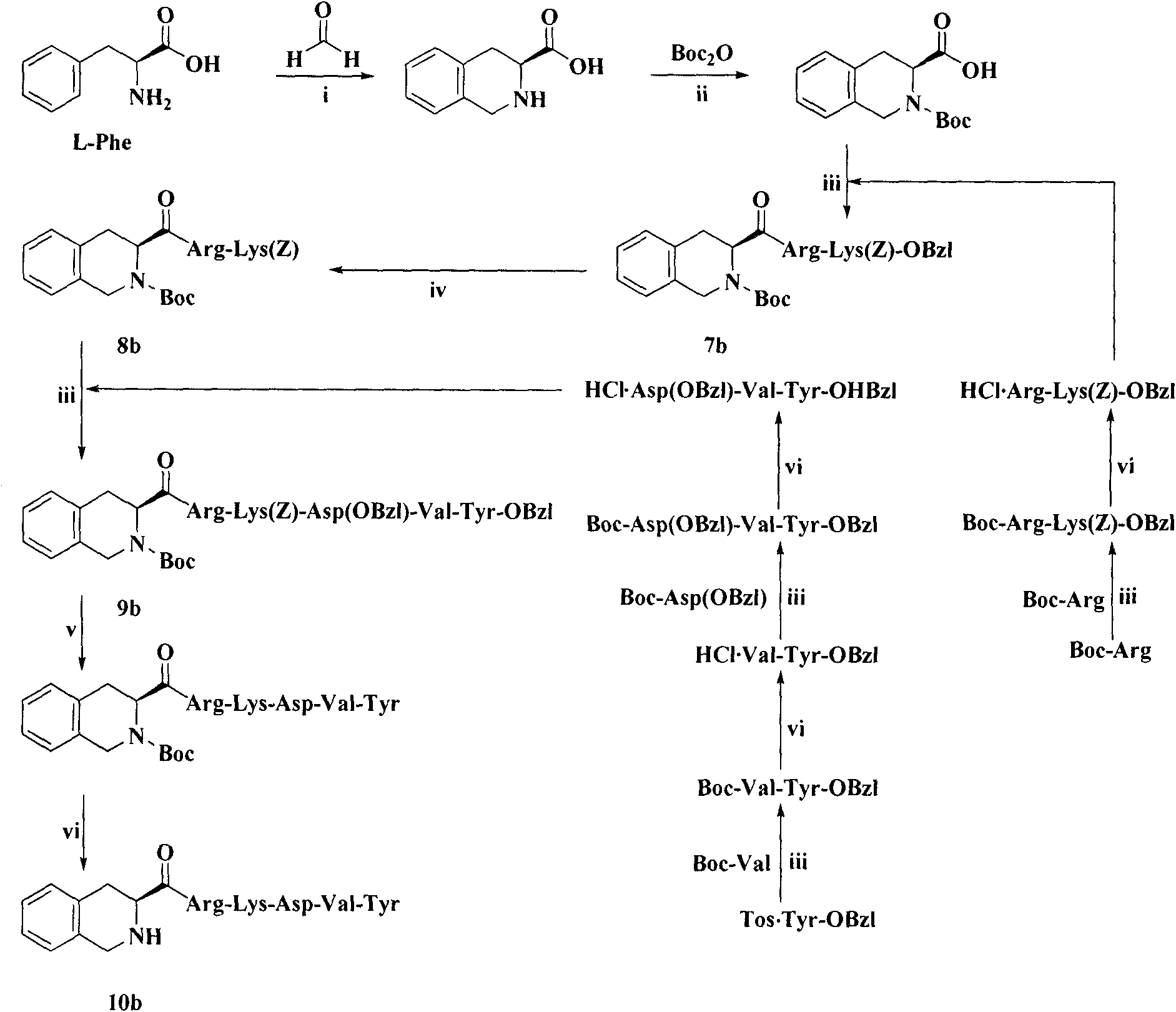
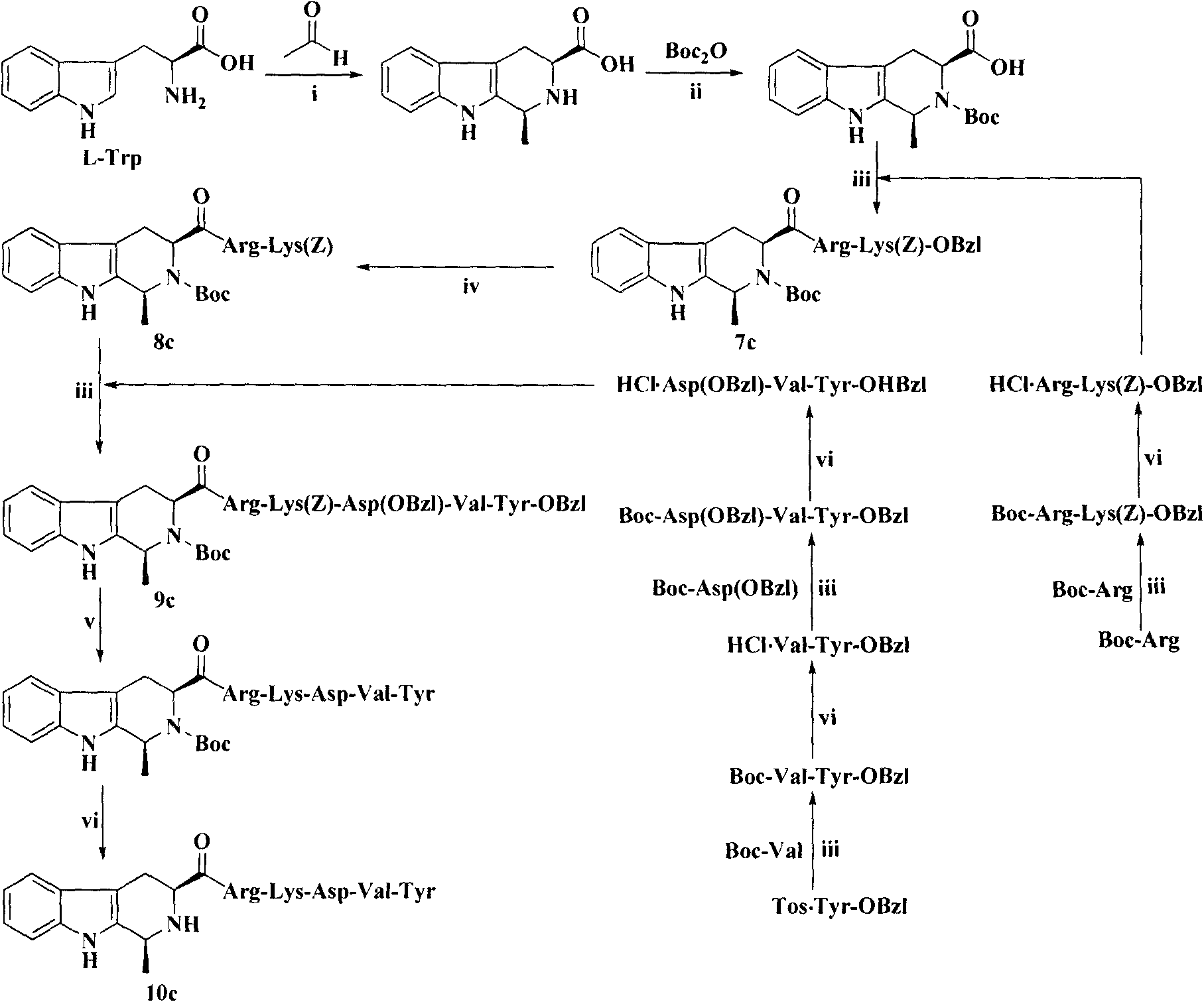
Image
Examples
Embodiment 1
[0073] Example 1 Preparation of Boc-Val-Tyr-OBzl (1)
[0074] Dissolve 0.434g (2.0mmol) Boc-Val in 15mL of anhydrous THF, and add 0.27g (2.0mmol) N-hydroxybenzotriazole (HOBt) and 0.45g (2.2mmol) dicyclohexyl Carbonyldiimide (DCC). After stirring for 5 minutes, a solution of 0.886 g (2.0 mmol) Tos.Tyr-OBzl in anhydrous THF was added. The reaction mixture was adjusted to pH 8-9 with N-methylmorpholine (NMM), and stirred in ice bath for 8 hours. The reaction was stopped, and the precipitated dicyclohexylurea (DCU) was removed by filtration. The filtrate was concentrated under reduced pressure, the residue was dissolved in ethyl acetate, and the resulting solution was successively washed with saturated NaHCO 3 Aqueous solution, saturated 5% KHSO 4 aqueous solution and saturated NaCl aqueous solution. Ethyl acetate layer with anhydrous NaSO 4 Let dry for 2 hours. Filter out NaSO 4 , and the filtrate was concentrated under reduced pressure to remove ethyl acetate. The resi...
Embodiment 2
[0075] Example 2 Preparation of HCl Val-Tyr-OBzl (2)
[0076] Dissolve 0.874g (1.79mmol) (1) in 10mL hydrogen chloride-ethyl acetate (4mol / L) solution, stir at room temperature for 2 hours, TLC detects that the raw material point disappears, remove ethyl acetate under reduced pressure, and add a small amount of ether repeatedly Vacuum under reduced pressure to remove acid gas from the product. Finally, a small amount of ether was added to grind the product into solid powder, which was directly used in the next reaction.
Embodiment 3
[0077] Example 3 Preparation of Boc-Asp(OBzl)-Val-Tyr-OBzl(3)
[0078] 0.578g (1.79mmol) Boc-Asp (OBzl) was dissolved in 15mL anhydrous THF, and 0.240g (1.78mmol) HOBt and 0.401g (1.95mmol) DCC were added under ice cooling. After stirring for 5 minutes, a solution of 0.758 g (1.79 mmol) HCl·Val-Tyr-OBzl (2) in anhydrous THF was added. The reaction mixture was adjusted to pH 8-9 with NMM, and stirred in an ice bath for 8 hours. The reaction was stopped, and the precipitated DCU was removed by filtration. The filtrate was concentrated under reduced pressure, the residue was dissolved in ethyl acetate, and the resulting solution was successively washed with saturated NaHCO 3 Aqueous solution, 5% KHSO 4 aqueous solution and saturated NaCl aqueous solution. Ethyl acetate layer with anhydrous NaSO 4 Let dry for 2 hours. Filter out NaSO 4 , and the filtrate was concentrated under reduced pressure to remove ethyl acetate. The residue was separated by column chromatography (CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com