Long-acting implantation agent of pentapeptide for thymus gland and method of producing the same
An implant and thymus technology, which is applied in the field of thymopentin long-acting implants and its preparation, can solve the problems of thymopentin, complex preparation process, and small molecular weight, etc., and achieve the elimination of burst release effect and simple preparation process , the effect of high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
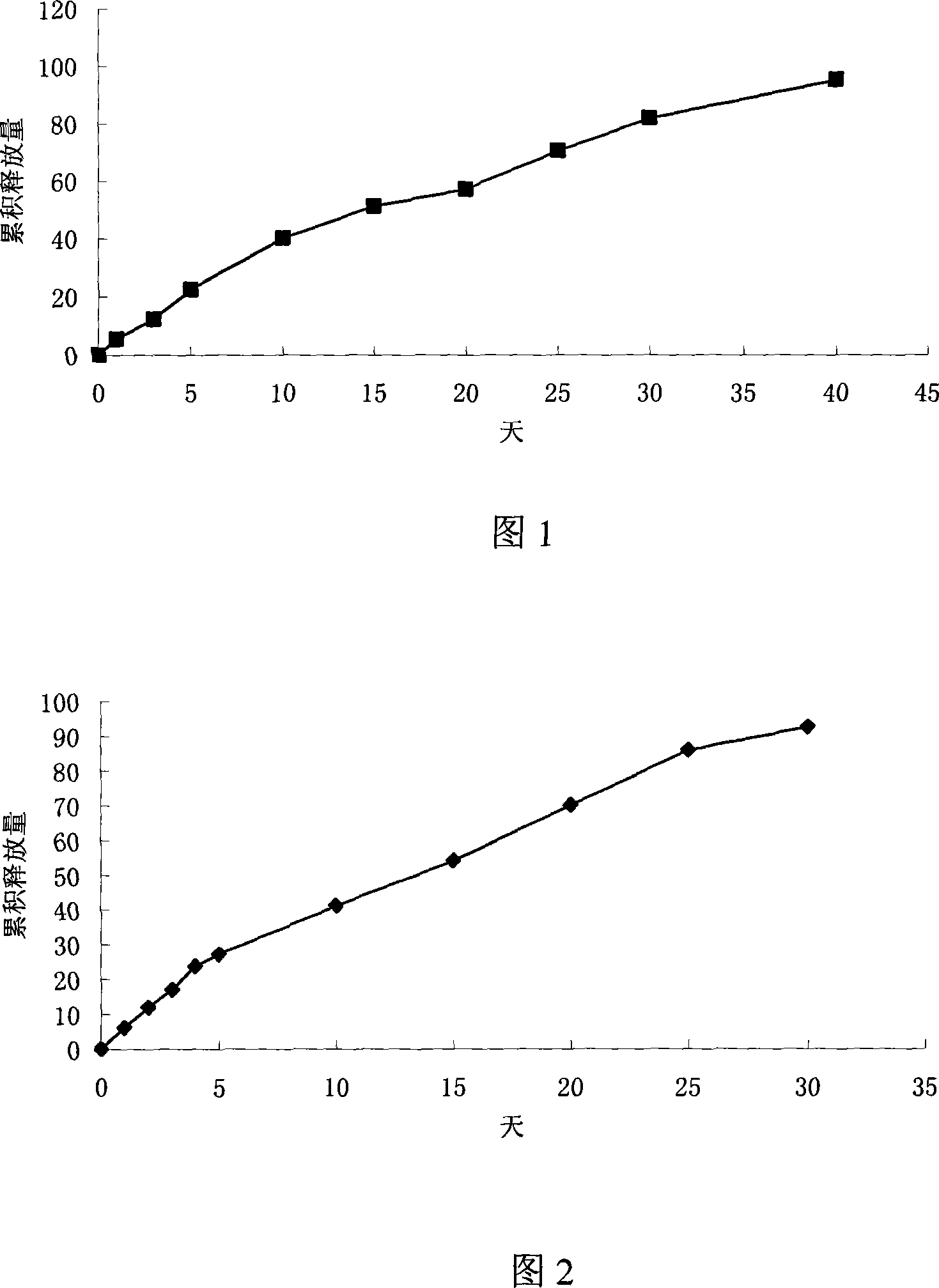
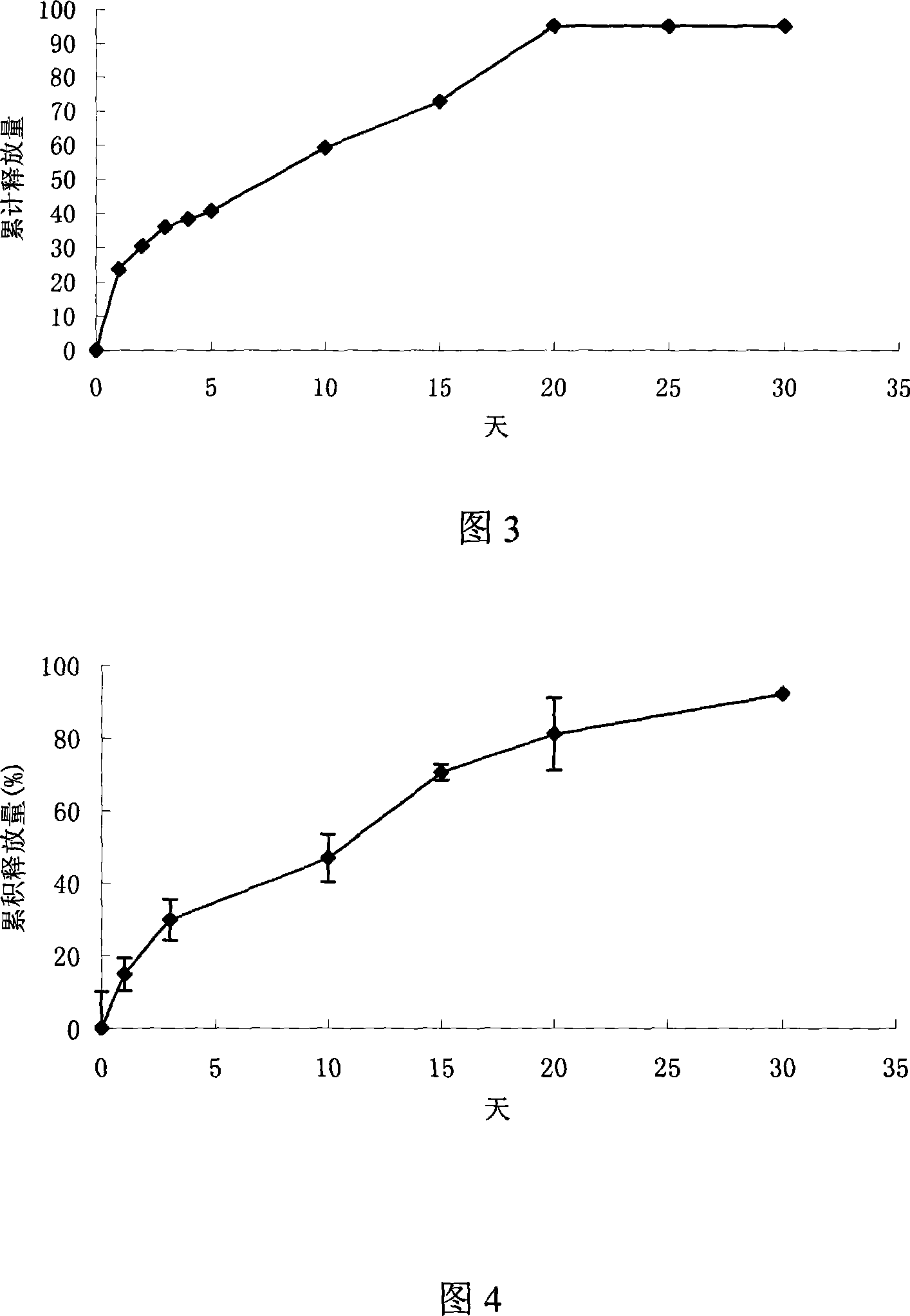
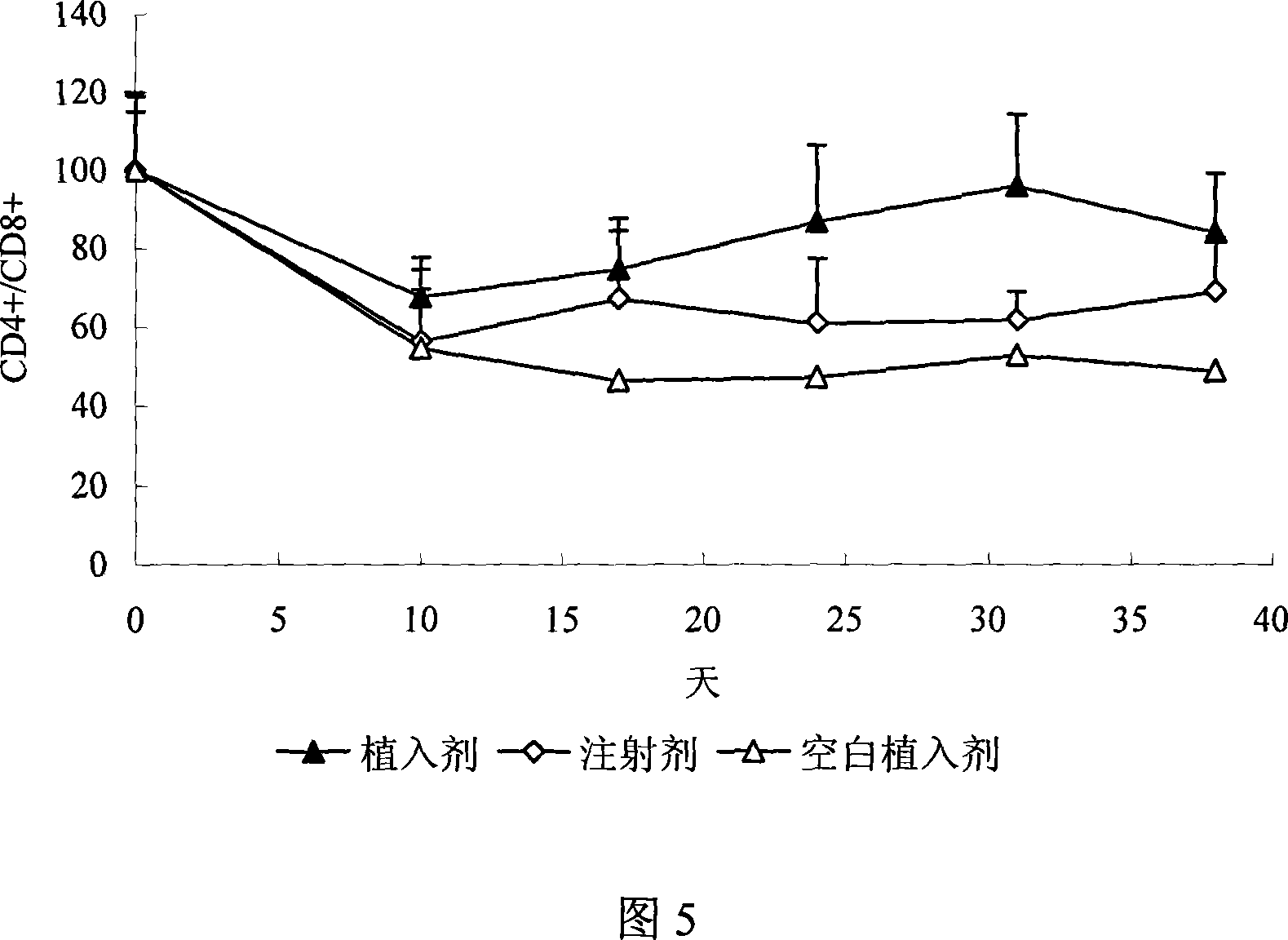
[0023] Weigh 797mg lactic acid-glycolic acid copolymer (PLGA, polymerization ratio 50:50, molecular weight 5000), 200mg polylactic acid (PLA, molecular weight 500000), dissolve in 4ml dichloromethane. Add 1mg Thymopentin, 1mg Mg(OH) 2 and 1 mg of polyethylene glycol, after uniform dispersion, volatilize at 30°C to remove part of the organic solvent, extrude the obtained semi-dry mixture, volatilize to remove the residual solvent, and obtain a cylindrical implant with a diameter of 0.5 mm and a length of 30 mm.
Embodiment 2
[0025] Weigh 545mg PLGA (polymerization ratio 50:50, molecular weight 15000), 448mg PLA (molecular weight 100000), heat to 80°C to soften and mix well, add 5mg thymopentin, 1mg ZnCO 3 and 1 mg of mannitol, stirred to disperse evenly, extruded under heat preservation conditions, and cooled to obtain a cylindrical implant with a diameter of 1 mm and a length of 20 mm.
Embodiment 3
[0027] Weigh 245mg PLGA (polymerization ratio 75:25, molecular weight 30000), 745mg PLA (molecular weight 60000), dissolve in 4ml dichloromethane, add 10mg thymopentin, disperse evenly, remove part of the organic solvent by volatilization at 25°C , the resulting semi-dry mixture was extruded, and the residual solvent was volatilized to remove a cylindrical implant with a diameter of 2 mm and a length of 10 mm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com