Composition of thymic peptide alpha 1 and thymopeptide-5 and preparation method thereof
A technology of composition and thymosin, which is applied in the field of composition of thymosin α1 and thymopentin, can solve the problems of low immune function, transfer, and low efficiency, and achieve rapid dissolution, good stability, and less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
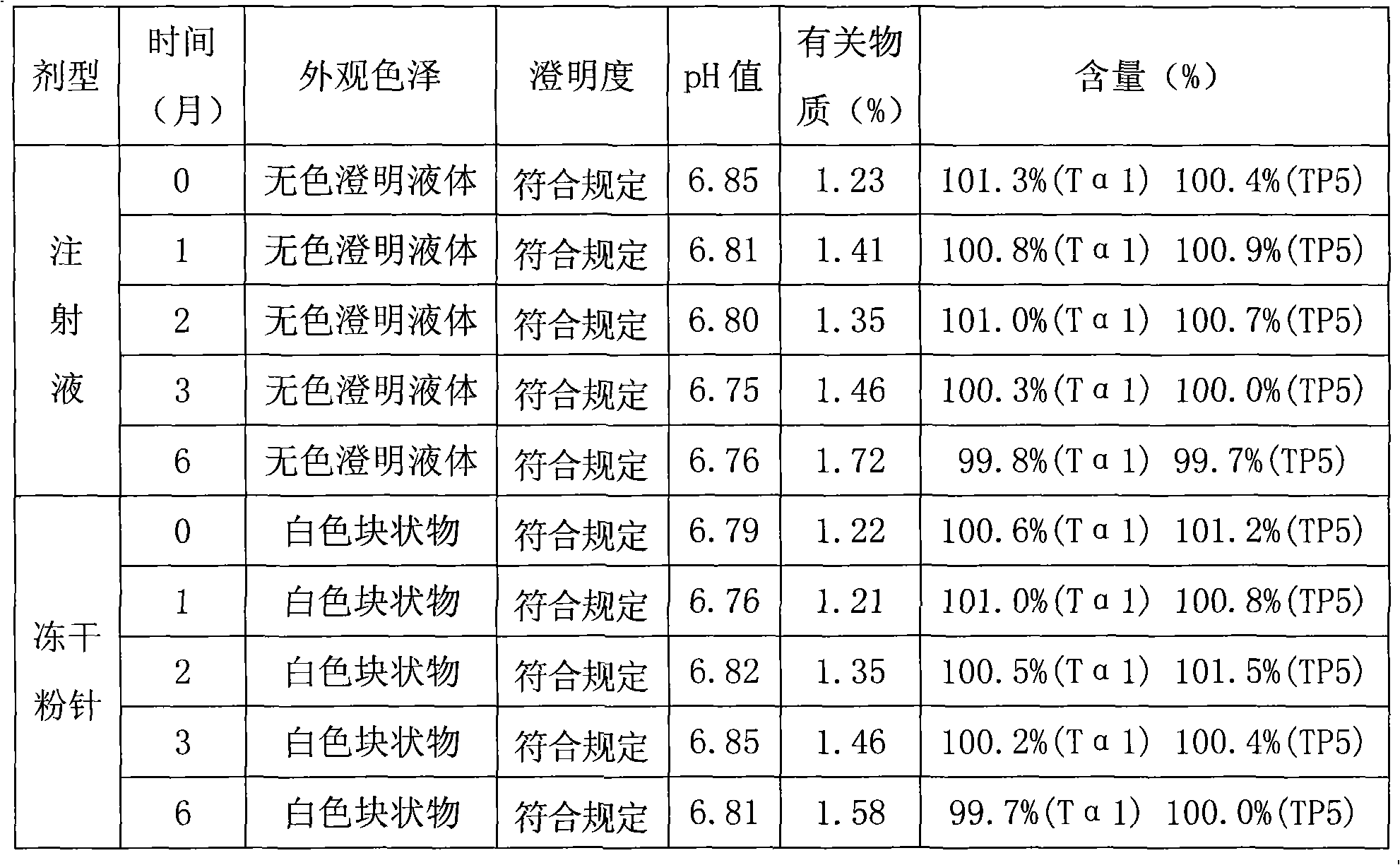
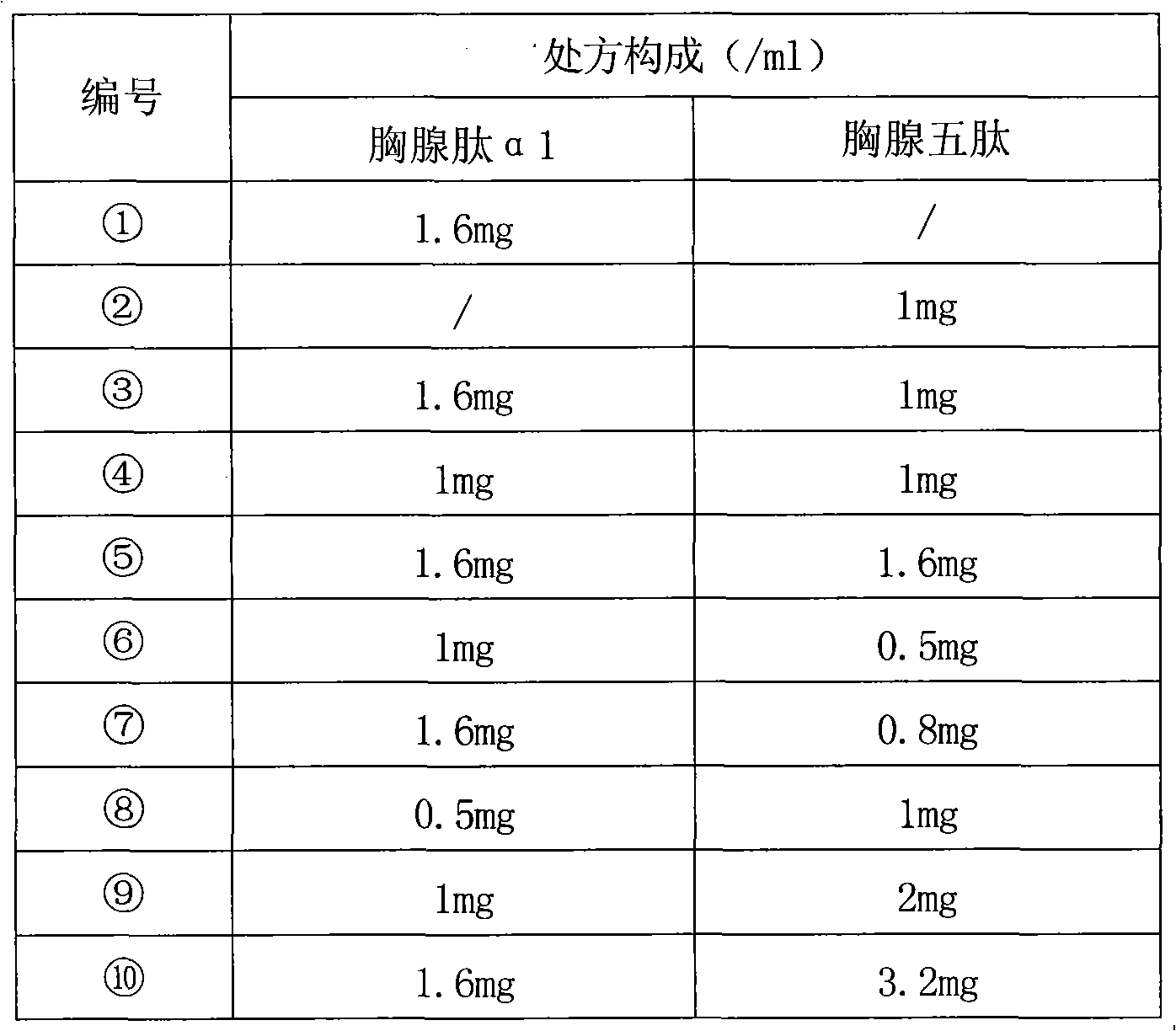
[0064] Example 1 Preparation of Thymosin α1 and Thymopentin Composition Injection
[0065] 1. Prescription
[0066] Prescription of Thymosin α1 and Thymopentin Composition (1)
[0067] Thymosin α1 1.6g
[0068] Thymopentin 1.0g
[0069] Mannitol 50g
[0070] Sodium dihydrogen phosphate 0.796g
[0071] Disodium hydrogen phosphate 1.755g
[0072] Add water for injection to 1000ml
[0073]
[0074] A total of 1000 bottles were made
[0075] Prescription of Thymosin α1 and Thymopentin Composition (2)
[0076] Thymosin α1 1.0g
[0077] Thymopentin 1.0g
[0078] Mannitol 25g
[0079] Sodium acetate 0.1g
[0080] Add water for injection to 1000ml
[0081]
[0082] Adjust the pH value to 5.5-7.5 with acetic acid and make a total of 1000 bottles
[0083] Prescription of Thymosin α1 and Thymopentin Composition (3)
[0084] Thymosin α1 1.6g
[0085] Thymopentin 1.6g
[0086]...
Embodiment 2
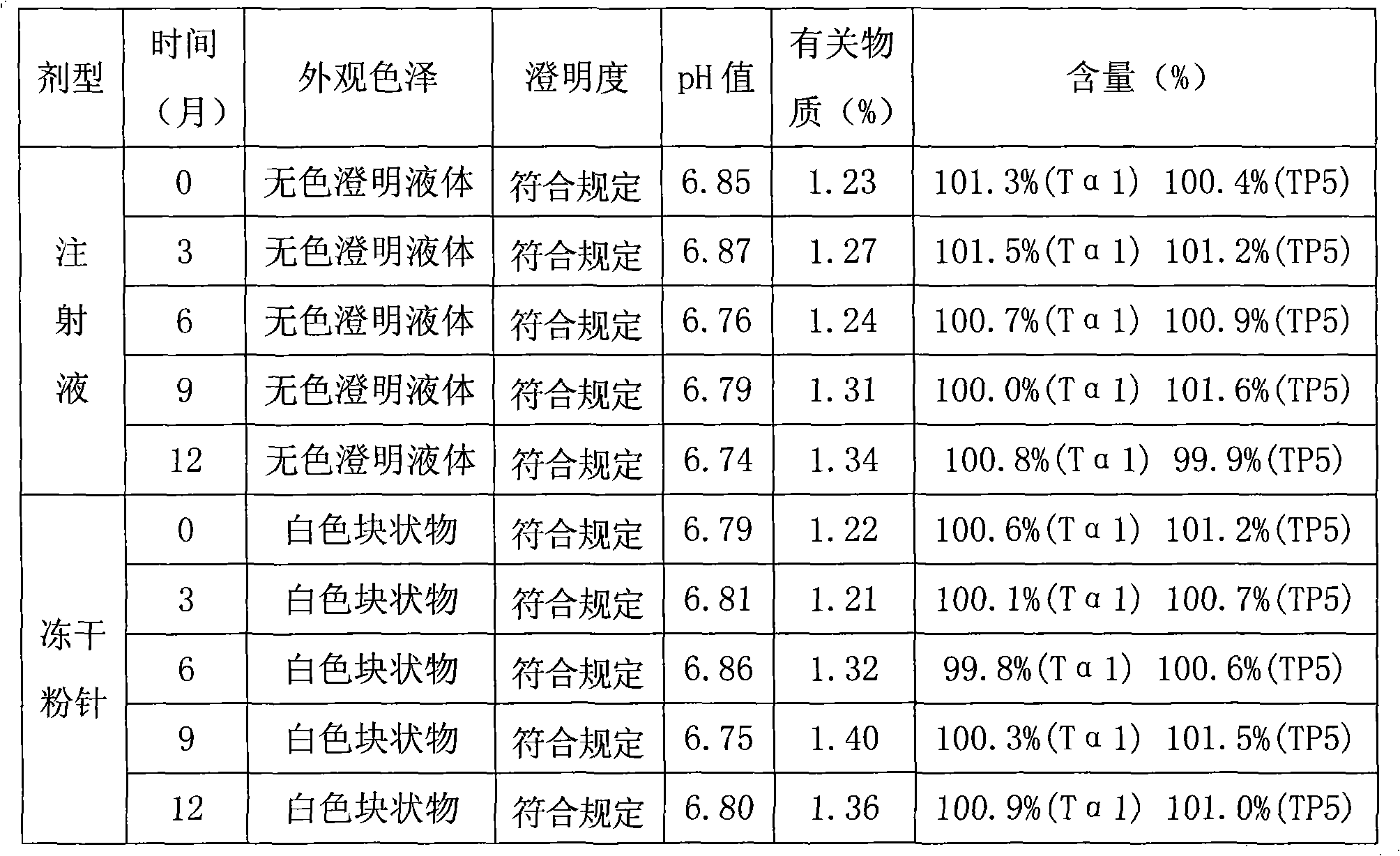
[0110] Embodiment 2 Preparation of thymosin α1 and thymopentin composition freeze-dried powder injection
[0111] 1. Prescription
[0112] Prescription of Thymosin α1 and Thymopentin Composition (1)
[0113] Thymosin α1 1.6g
[0114] Thymopentin 0.8g
[0115] Mannitol 50g
[0116] Sodium dihydrogen phosphate 0.796g
[0117] Disodium hydrogen phosphate 1.755g
[0118] Add water for injection to 1000ml
[0119]
[0120] A total of 1000 bottles were made
[0121] Prescription of Thymosin α1 and Thymopentin Composition (2)
[0122] Thymosin α1 0.5g
[0123] Thymopentin 1.0g
[0124] Mannitol 25g
[0125] Sodium acetate 0.1g
[0126] Add water for injection to 1000ml
[0127]
[0128] Adjust the pH value to 5.5-7.5 with acetic acid and make a total of 1000 bottles
[0129] Prescription of Thymosin α1 and Thymopentin Composition (3)
[0130] Thymosin α1 1.0g
[0131] Thymopentin 2....
Embodiment 3
[0154] The preparation of embodiment 3 thymosin alpha 1 and thymopentin composition
[0155] The dose of thymosin α1 is 0.1 mg; the dose of thymopentin is 0.1 mg.
[0156] The preparation method is the same as claim 1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com