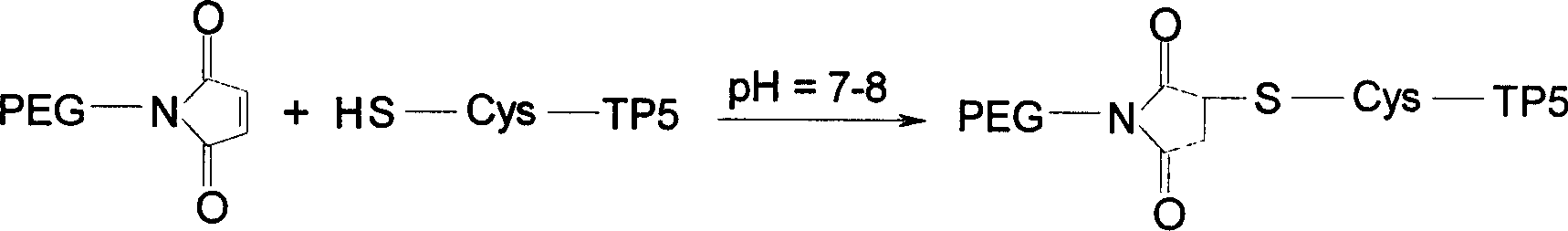Carbowax alcoholized ramification of TimopEntin, combination of medication and application
A PEGylation and polyethylene glycol technology, applied in the field of compounds, can solve problems such as the lack of thymopentin, and achieve the effects of novel chemical structure, good application prospects and stable biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 mPEG 5000 -NHCOCH 2 CH 2 Synthesis of CO-TP5
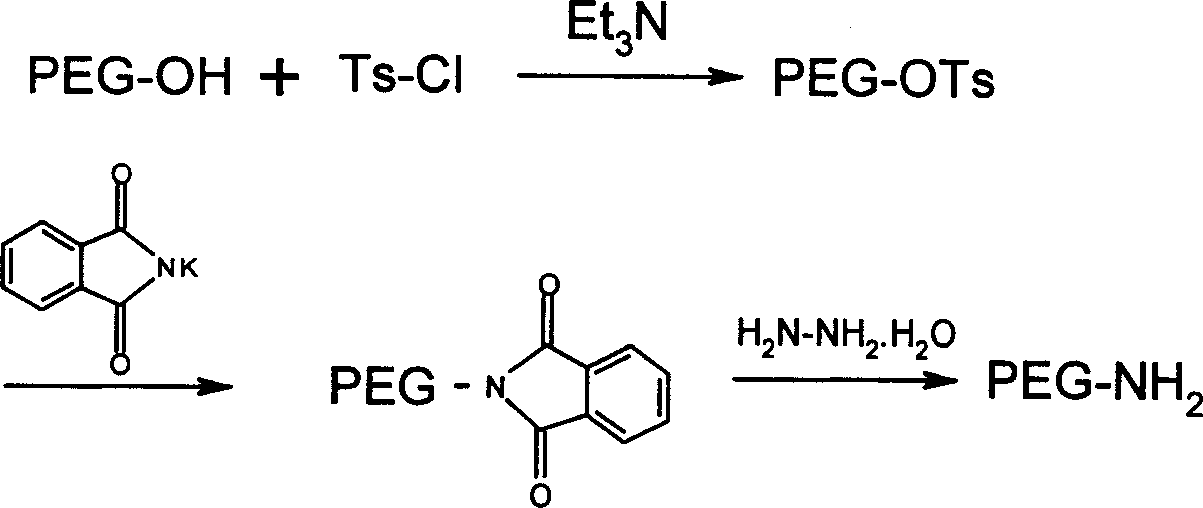
[0045] Weigh mPEG 2000 -OH 40.0g (20mmol) was placed in a 250ml reaction bottle, and 100ml CH was added 2 Cl 2 , after the solid dissolves, add 15mlEt 3 N (100mmmol) and 19.0g Ts-Cl (100mmol) were reacted with stirring at room temperature. After TLC monitors that the reaction is complete, the solvent is removed by rotary evaporation, and 100ml of anhydrous ether is added to precipitate a solid to obtain 28.5gmPEG 2000 -OTs, yield 71%
[0046] 18.0gmPEG 2000 -OTs (9 mmol) was dissolved in 50 ml of DMF, 5.0 g (27 mmol) of phthalimide potassium salt was added, and reacted at 120° C. for 4 hours. The solvent was distilled off under reduced pressure, the residue was dissolved in 50ml of absolute ethanol, 13.5ml of hydrazine hydrate was added, and the mixture was refluxed for 4 hours. The solvent was removed by rotary evaporation and the residue was dissolved in CH 2 Cl 2 , Precipitate the solid wit...
Embodiment 2
[0050] Implementation of 2 mPEG 5000 -NHCOCH 2 CH 2 Synthesis of CO-TP5
[0051] Weigh mPEG 2000 -OH 50.0g (10mmol) was placed in a 250ml reaction bottle, and 50ml CH was added 2 Cl 2 , the solid dissolved and then added 7.5ml Et 3 N (50mmmol) and 9.5g Ts-Cl (50mmol), stirred at room temperature. After TLC monitors that the reaction is complete, the solvent is removed by rotary evaporation, and 100ml of anhydrous ether is added to precipitate a solid to obtain 33.5gmPEG 5000 -OTs, yield 67%
[0052] 30.0g mPEG 5000 -OTs (6mmol) was dissolved in 30ml of DME, 3.33g (18mmol) of phthalimide potassium salt was added, and reacted at 120°C for 4 hours. The solvent was distilled off under reduced pressure, the residue was dissolved in 50ml of absolute ethanol, 4.0ml of hydrazine hydrate was added, and the mixture was refluxed for 4 hours. The solvent was removed by rotary evaporation and the residue was dissolved in CH 2 Cl 2 , Precipitate the solid with anhydrous et...
Embodiment 3
[0056] Implementation of 3 Cys (mPEG 2000 Synthesis of -MAL)-TP5
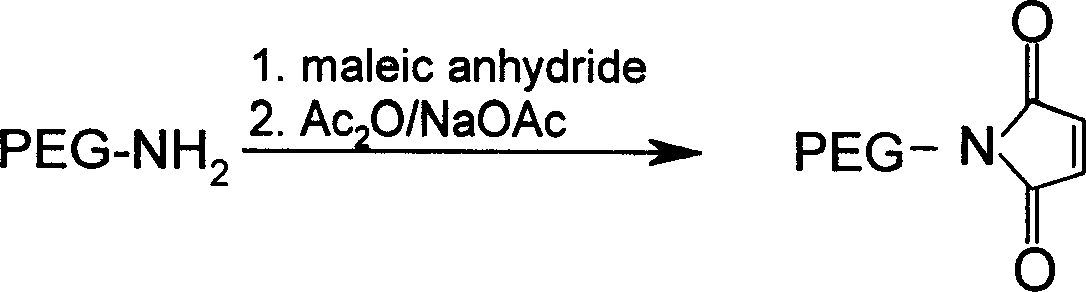
[0057] 5.0gmPEG 2000 -NH 2 Dissolve in 10ml of dioxane, add 2.0g of maleic anhydride, stir and react at 80°C for 30min. The solvent was evaporated under reduced pressure, 50ml of anhydrous diethyl ether was added, the solid was precipitated by cooling, and the solid was collected by filtration and dried to obtain 4.9g. The obtained solid was dissolved in 15ml of acetic anhydride, 5.0g of sodium acetate was added, and the reaction was stirred at 100°C for 45min. Evaporate the solvent under reduced pressure, dissolve the residue with dichloromethane, filter off the insoluble matter, add an appropriate amount of activated carbon to the filtrate, let it stand for 30 minutes, filter out the activated carbon, concentrate the filtrate to dryness, add anhydrous ether, precipitate a solid, filter After collection and drying, light yellow solid 2.5gmPEG was obtained 2000 -MAL, yield 50%.
[0058] With 100mg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com