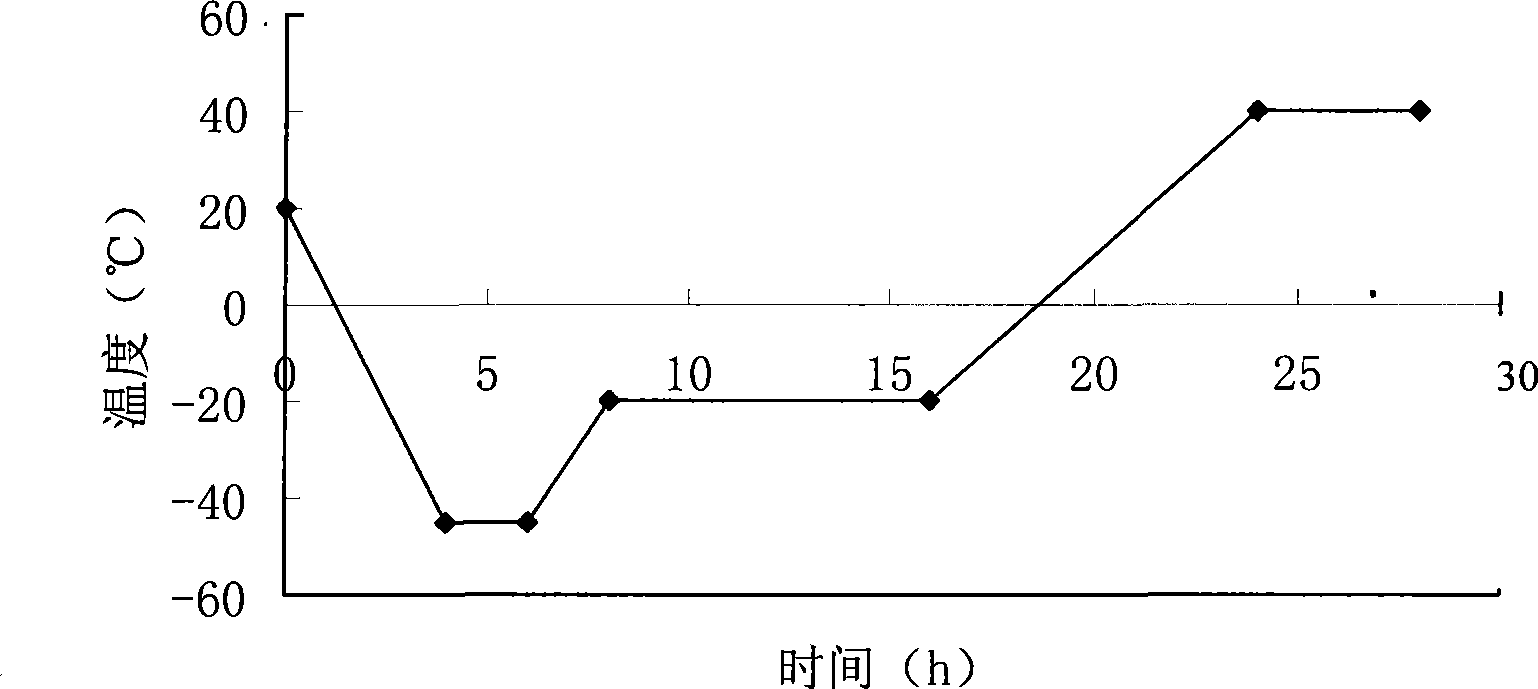
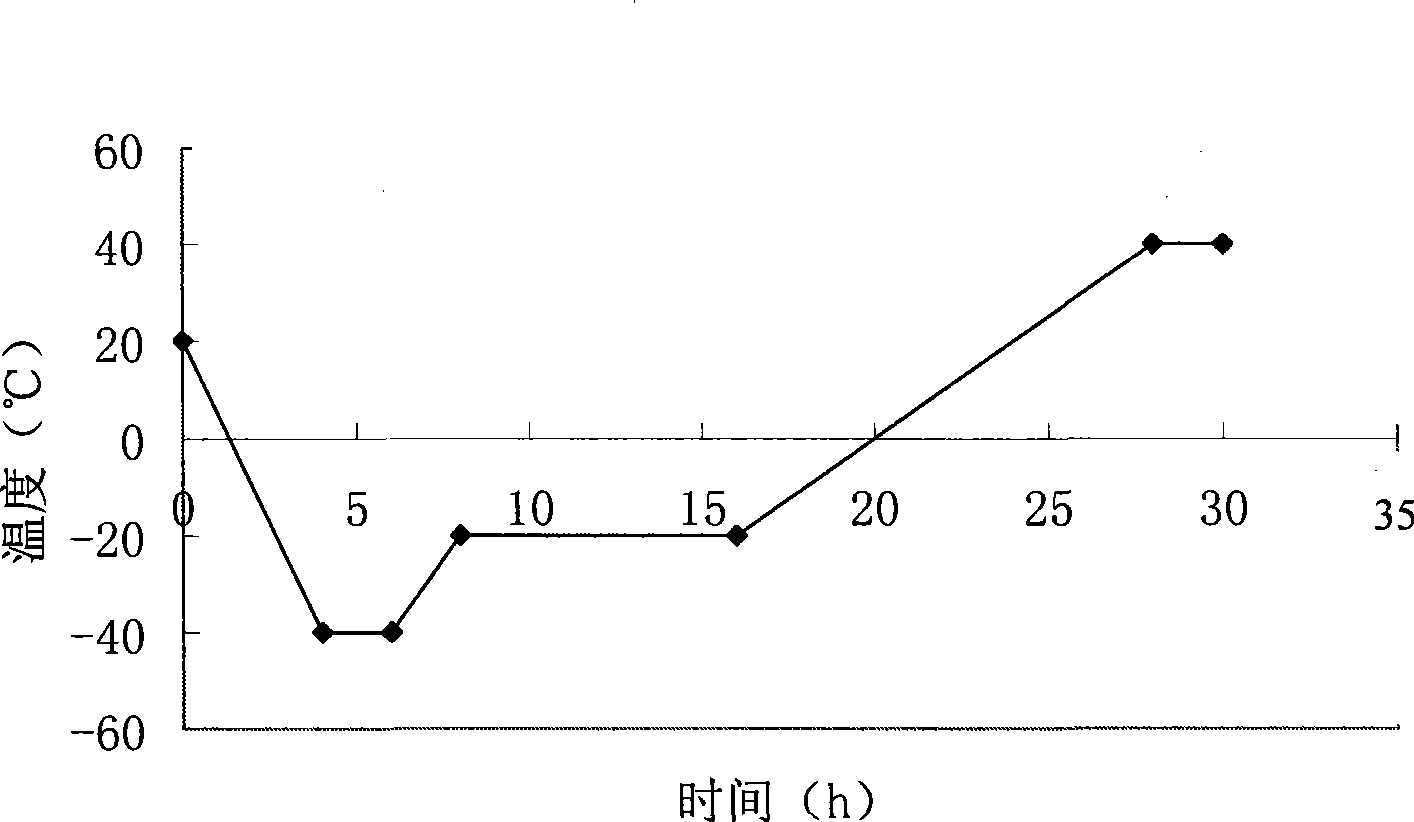
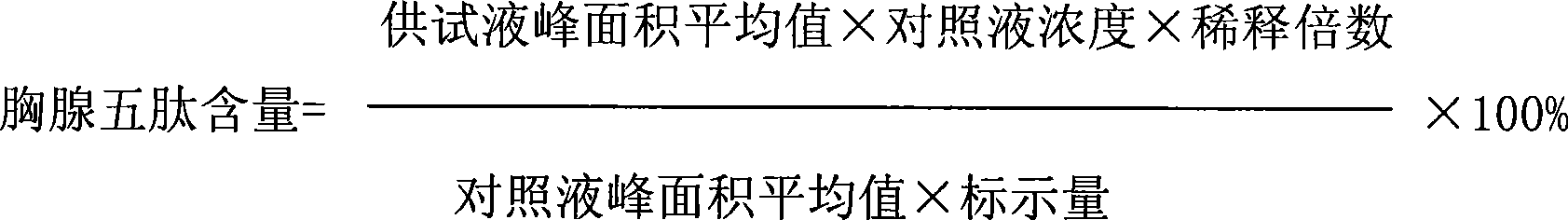
Thymopentin dry powder formulation and preparation method thereof
A technology of dry powder preparation and thymus, which is applied in the field of thymopentin dry powder preparation and its preparation, can solve the problems of poor stability of peptide bonds of Asp residues, inability to remove, and inability to completely remove impurities, etc., to achieve good solubility, curative effect and safety sex-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, specification is the preparation of the thymopentin dry powder preparation of 10mg
[0052] 1. Preparation:
[0053] 1. Measure 800ml of water for injection at 35°C, accurately weigh 30g of mannitol, dissolve the above-mentioned 30g of mannitol in the above-mentioned 800ml of water for injection, and mix well to obtain solution I; add activated carbon for needles (Shanghai Activated Carbon Factory) to solution I , activated carbon for needles accounted for 0.05% of the solution I mass, stirred at room temperature for 30 minutes, then decarbonized with a 0.45 μm microporous membrane filter, and collected the filtrate I;
[0054] Accurately weigh 10g of thymopentin, dissolve it in all the above-mentioned filtrates I, and mix well to obtain solution II; adjust the pH value of solution II to 7.0 with 0.1mol / L sodium dihydrogen phosphate solution, and make up solution II to 1000ml with water for injection ; Filter the solution II as follows in turn: filter thr...
Embodiment 2
[0095] Embodiment 2, the preparation of the Thymopentin dry powder preparation that specification is 1mg
[0096] 1. Preparation:
[0097] 1. Measure 400ml of water for injection at 37°C, accurately weigh 30g of mannitol, dissolve the above 30g of mannitol in the above 400ml of water for injection, and mix well to obtain solution I; add activated carbon for needles (Shanghai Activated Carbon Factory) to solution I , activated carbon for needles accounted for 0.05% of the solution I mass, stirred at room temperature for 30 minutes, then decarbonized with a 0.45 μm microporous membrane filter, and collected the filtrate I;
[0098] Accurately weigh 1g of thymopentin, dissolve it in all the above-mentioned filtrates I, and mix well to obtain solution II; adjust the pH value of solution II to 7.1 with 0.1mol / L sodium dihydrogen phosphate solution, and make up solution II to 500ml with water for injection ; Filter the solution II in sequence as follows: filter through a 0.22 μm mi...
Embodiment 3
[0133] Embodiment 3, specification is the preparation of the dry powder preparation of thymopentin of 10mg
[0134] 1. Preparation:
[0135] 1. Measure 800ml of water for injection at 40°C, accurately weigh 10g of lactose, dissolve the above 10g of lactose in the above 800ml of water for injection, and mix well to obtain solution I; add activated carbon for needles (Shanghai Activated Carbon Factory) to solution I, and inject Use activated carbon to account for 0.05% of the mass of solution I, stir at room temperature for 20 minutes, then filter and decarbonize with a 0.45 μm microporous membrane, and collect filtrate I;
[0136] Accurately weigh 10g of thymopentin, dissolve it in all the above filtrates I, and mix well to obtain solution II; adjust the pH value of solution II to 7.2 with 0.2mol / L sodium dihydrogen phosphate solution, and make up solution II to 1000ml with water for injection ; Filter the solution II as follows in turn: filter through a 0.22 μm microporous me...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com