Pentapeptide nose spraying agent for thymus gland, preparation method and application thereof
A thymus and preparation technology, which is applied in the direction of aerosol delivery, anti-inflammatory agents, antiviral agents, etc., can solve the problems of injection site infection, patient injection pain, etc., and achieve the effects of long storage time, low cost, and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The prescription is as follows:
[0078] Thymopentin: 25.0g
[0080] Citric acid monohydrate: 1.7g
[0081] Disodium hydrogen phosphate dihydrate: 3.0g
[0082] Benzalkonium chloride (50%): 0.2g
[0083] Add water for injection to: 1250ml
[0084]
[0085] Adjust the pH to 4.0-5.5 and make a total of 500 bottles
[0086] The preparation method of preparation of the present invention is as follows:
[0087] 1. Weigh the above-mentioned raw and auxiliary materials in the prescription amount into the sterilization container in the 100,000-class clean area;
[0088] 2. After adding an appropriate amount of water for injection, stir well until it is completely dissolved;
[0089] 3. Adjust the pH value to between 4.0 and 5.0 with 0.1M hydrochloric acid or sodium hydroxide;
[0090] 4. Add water for injection to the full amount;
[0091] 5. Transfer to the clean area of 10,000 grades, and fin...
Embodiment 2
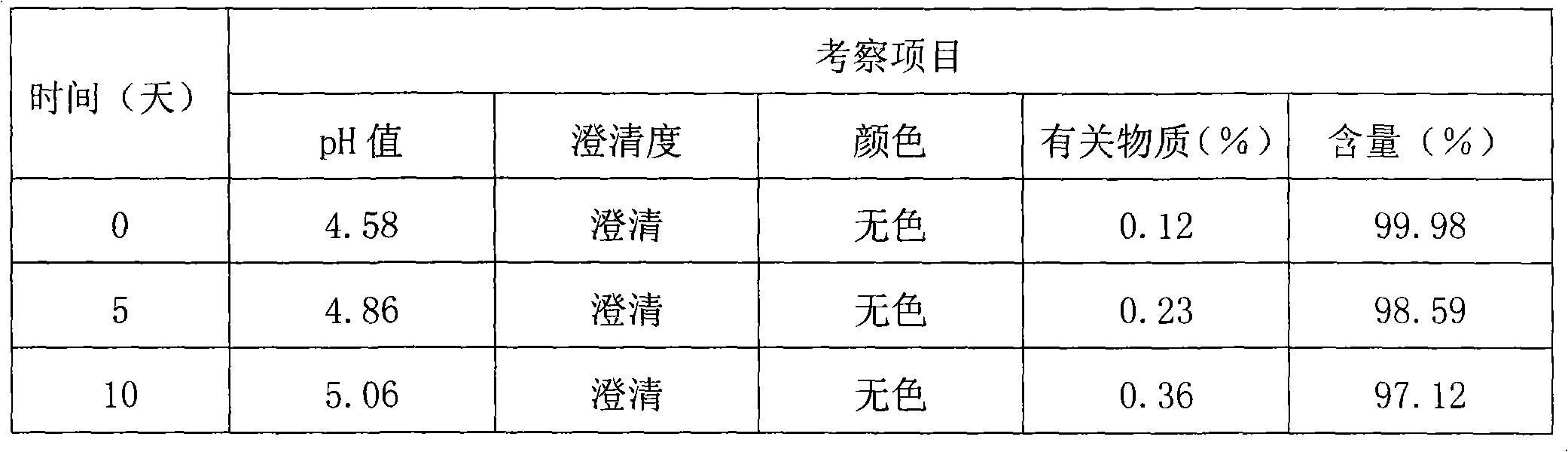
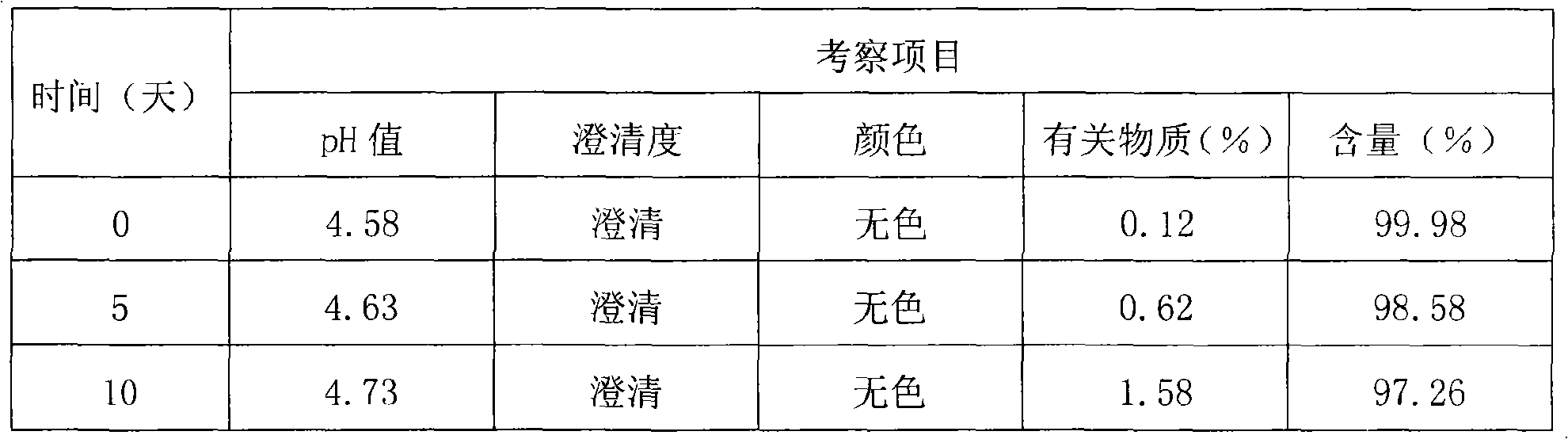
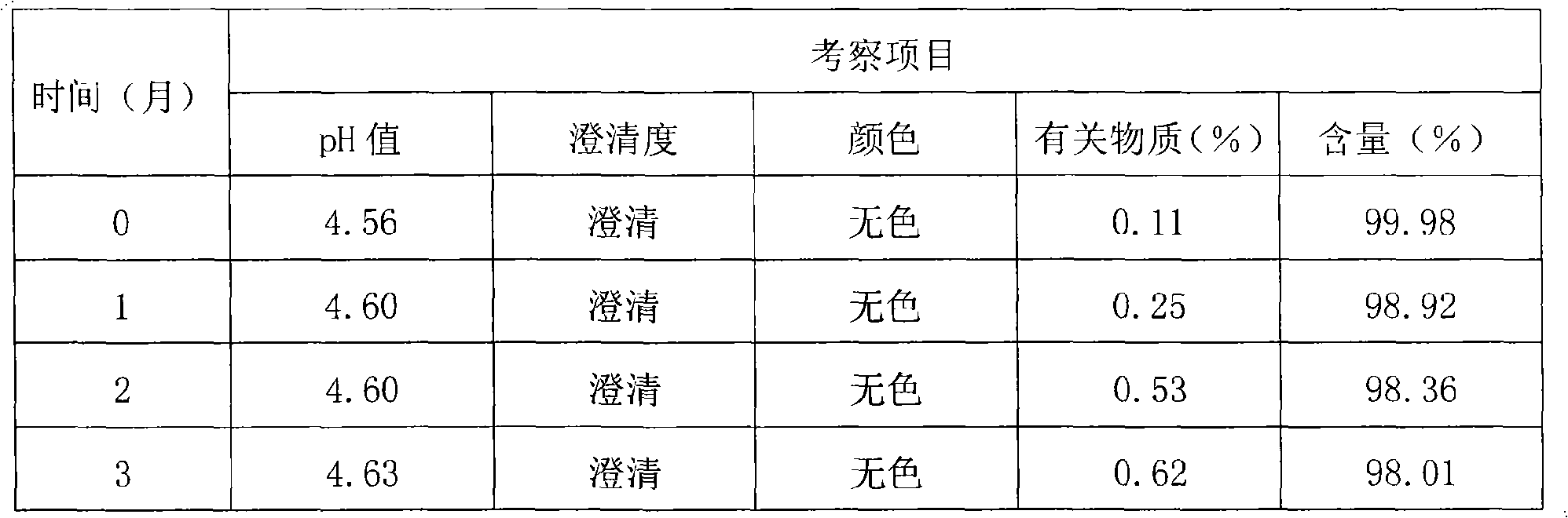
[0095] quality control method
[0096] Thymopentin Nasal Spray
[0097] Xiongxianwutai Bipenwuji
[0098] Thymopentin Nasal Spray
[0099] This product is an aqueous solution of thymopentin. In terms of peptide content, it contains thymopentin (C 30 h 49 N 9 o 9 ) should be 90.0-110.0% of the marked amount.
[0100] 【Properties】This product is a colorless clear liquid.
[0101] 【identification】
[0102] (1) Take about 1mg of this product, add 1ml of water to dissolve, add biuret reagent (take 0.15g of copper sulfate, add 0.6g of potassium sodium tartrate, add 50ml of water, add 30ml of 10% sodium hydroxide solution under stirring, add 100ml of water , that is) 1ml, that is blue-purple.
[0103] (2), in the chromatogram recorded under the assay item, the retention time of the main peak of the test product should be consistent with the retention time of the main peak of the reference substance.
[0104] 【an examination】
[0105] The pH value of this product was deter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com