Thymus pentapeptide slow-release microshpere formulation for injection and its preparation method
A technology for sustained-release microspheres and injections, which can be used in medical preparations containing active ingredients, antiviral agents, pharmaceutical formulations, etc., and can solve the problems of low bioavailability, large toxic and side effects, and poor patient compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: Thymopentin Microspheres Prepared by Double Emulsion Method
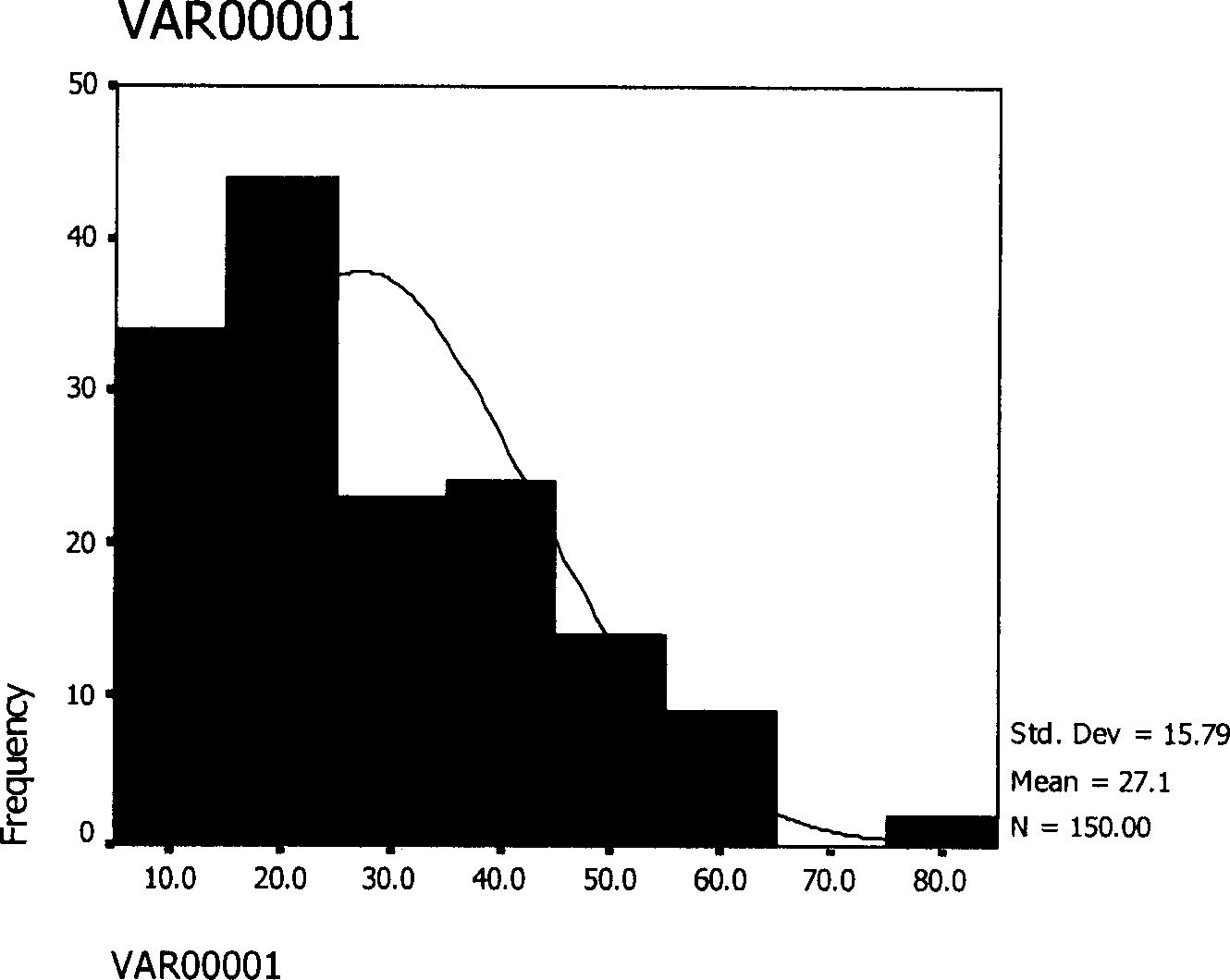
[0057] 50mg thymopentin was dissolved in 0.5ml gelatin aqueous solution, 500mg polylactide-glycolide (lactide:glycolide=50:50) (molecular weight is 11000) was dissolved in 5ml dichloromethane, the two Combined, the high-speed homogenizer was stirred at a high speed of 10,000 rpm for 3 minutes to form a W / O emulsion, and the W / O emulsion was poured into 500ml of the external water phase containing 0.2% polyvinyl alcohol to form a W / O / W double emulsion. Stir at a low speed of 500 rpm for 4 hours, volatilize the organic solvent, centrifuge, wash 3 times with distilled water, and freeze-dry for 20 hours to obtain a white powder with good fluidity, the encapsulation rate is 76%, and the particle size range of microspheres is 5~ 80μm, the average particle size is 27.1μm, and the appearance is round, such as figure 1 shown.
Embodiment 2
[0058] Embodiment 2: Double emulsion method prepares thymopentin microspheres
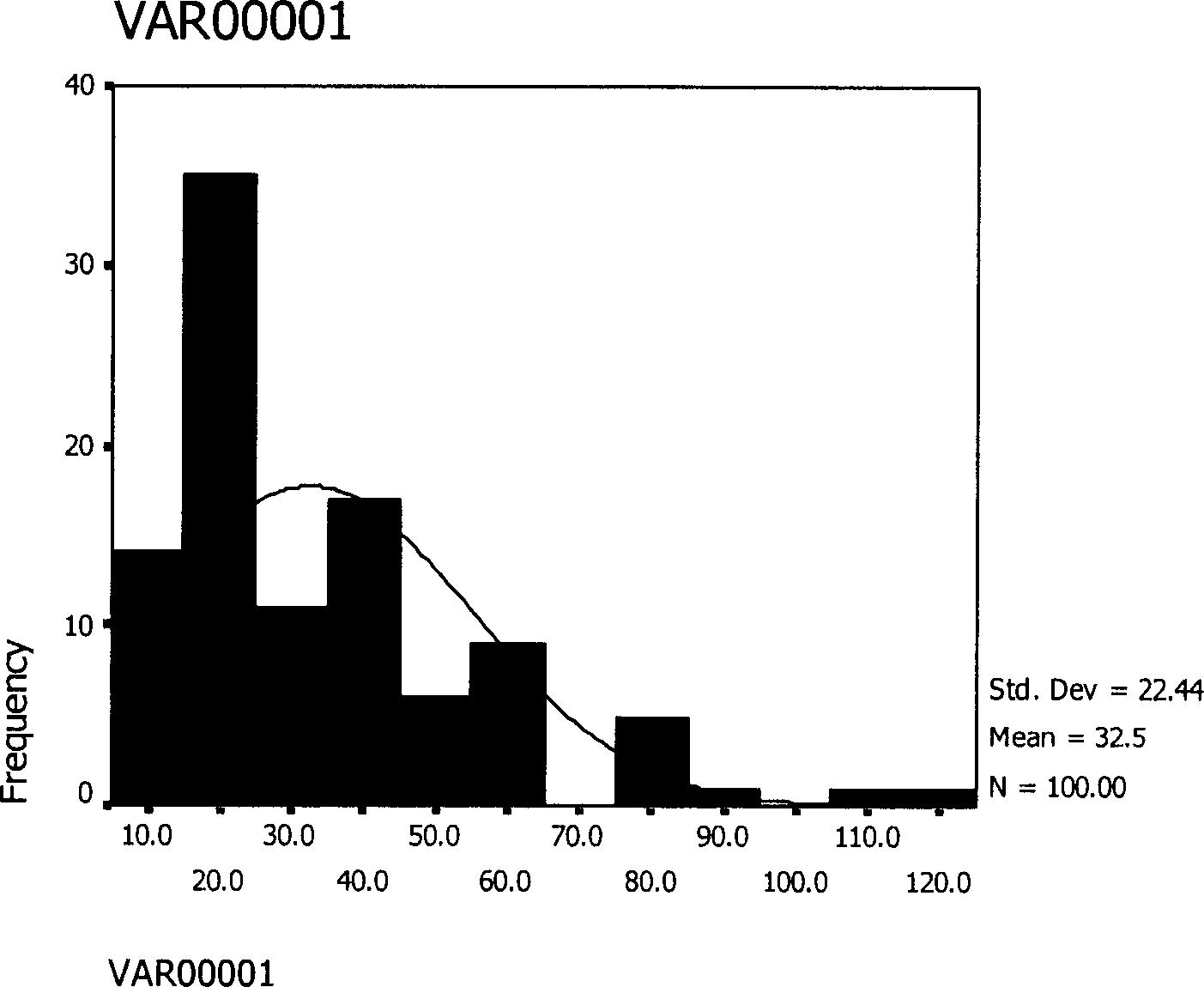
[0059] 100mg thymopentin was dissolved in 1.5ml gelatin aqueous solution, 1.0g polylactide-glycolide (lactide:glycolide=75:25) (molecular weight is 11000) was dissolved in 12ml dichloromethane, dichloromethane The two are combined, and the high-speed homogenizer is stirred at a high speed of 10,000 rpm for 5 minutes to form a W / O emulsion, and the W / O emulsion is poured into 1000ml of the external water phase containing 0.2% polyvinyl alcohol to form a W / O / W double emulsion , stirred at a low speed of 500 rpm for 4 hours, volatilized the organic solvent, centrifuged, washed 3 times with distilled water, and freeze-dried for 20 hours to obtain a white powder with good fluidity. The encapsulation rate was 76%, and the measured particle size was 5~ 120μm, average particle size 32.5μm, such as figure 2 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com