Drug composition of thymopentin composed of five kinds of amino acids and preparation method thereof
A technology of composition and thymus, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of easy hydrolytic breakage of peptide bonds, easy breakage, and short storage time of thymopentin preparations And other problems, to achieve the effect of high stability, good quality and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of thymopentin injection with a specification of 1ml:1mg
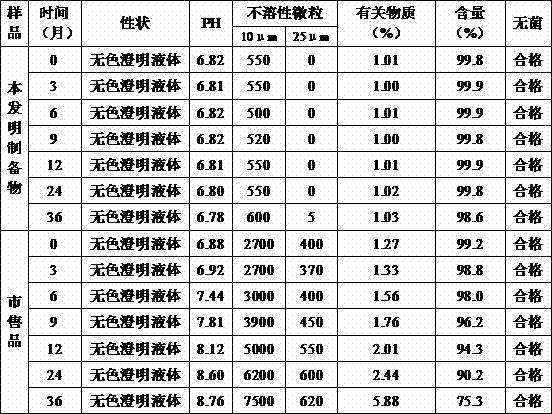
[0043] Take 0.5g of citric acid and 1.5g of dipotassium hydrogen phosphate into 400g of water for injection, stir and dissolve, then add 0.5g of sodium tartrate and 50g of glucose, stir and dissolve; add water for injection to 900g, add 1g of thymopentin, stir and dissolve, Add the remaining 100g of water for injection, stir evenly, add 0.15% (g / ml) of activated carbon for needles, stir for 25 minutes, filter and decarbonize, check the intermediates, filter and sterilize the filtrate with a 0.22μm filter membrane after passing the test; press the filtrate to 1ml The specifications are poured into an ampoule bottle, filled with nitrogen, melted and sealed, and after passing the inspection, packaged to obtain the thymopentin pharmaceutical composition.
Embodiment 2
[0044] Example 2 Preparation of thymopentin injection with specifications of 1ml:10mg
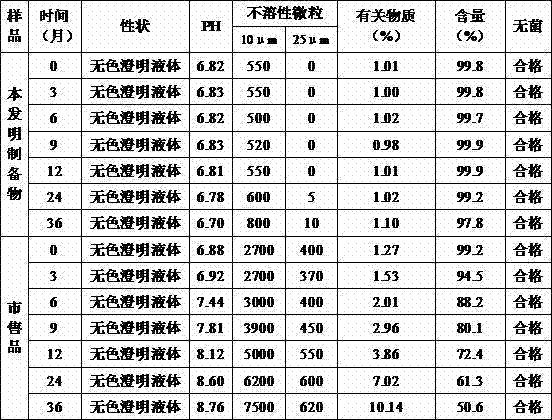
[0045] Take 0.5g of citric acid and 1.5g of dipotassium hydrogen phosphate into 400g of water for injection, stir and dissolve, then add 0.5g of sodium tartrate and 50g of glucose, stir and dissolve; add water for injection to 900g, add 10g of thymopentin, stir and dissolve, Add the remaining 100g of water for injection, stir evenly, add 0.15% (g / ml) of activated carbon for needles, stir for 25 minutes, filter and decarbonize, check the intermediates, filter and sterilize the filtrate with a 0.22μm filter membrane after passing the test; press the filtrate to 1ml The specifications are poured into an ampoule bottle, filled with nitrogen, melted and sealed, and after passing the inspection, packaged to obtain the thymopentin pharmaceutical composition.
[0046]
Embodiment 3
[0047] Example 3 Preparation of thymopentin for injection with a specification of 1 mg
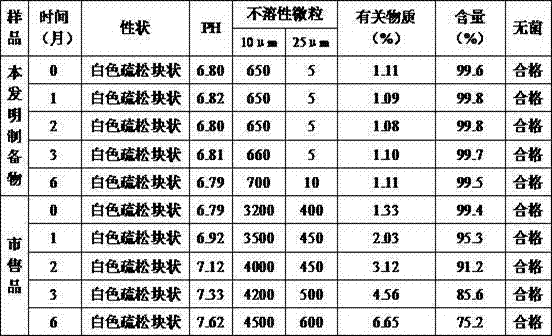
[0048]Take 0.5g of citric acid and 1.5g of dipotassium hydrogen phosphate into 400g of water for injection, stir and dissolve, then add 0.5g of sodium tartrate and 50g of glucose, stir and dissolve; add water for injection to 900g, add 1g of thymopentin, stir and dissolve, Add the remaining 100g of water for injection, stir evenly, add 0.15% (g / ml) of activated carbon for needles, stir for 25 minutes, filter and decarbonize, check the intermediates, filter and sterilize with a 0.22μm filter membrane after passing the test; freeze drying: ① Pre-freezing: Put about 1ml of sterilization solution into a freezer that has been pre-cooled to -20°C, keep it for 1 hour, cool down at a constant speed (≤1.5°C / min) to -40°C, and keep it warm for 2 hours; ②Sublimation: turn on the vacuum Device, control the vacuum degree to 12Pa, heat up at a constant rate (≤1.5℃ / min) to -18℃, keep at this temperature ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com