Slow releasing microsphere preparation of Thymopentin, and prepartion method
A slow-release microsphere preparation and thymus technology, which is applied in pharmaceutical formulations, peptide/protein components, medical preparations containing active ingredients, etc., can solve problems such as low bioavailability, different courses of treatment, and loss of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
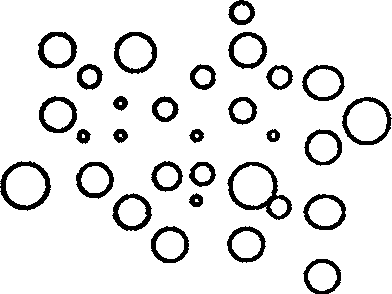
[0026] exist figure 1 In the process, the O phase is weighed according to the proportion and added to the W1 phase, and the crusher is used for thorough crushing. Through microscopic observation, the formation of figure 1 The transparent unstable spherical microspheres in the W1 / O phase.
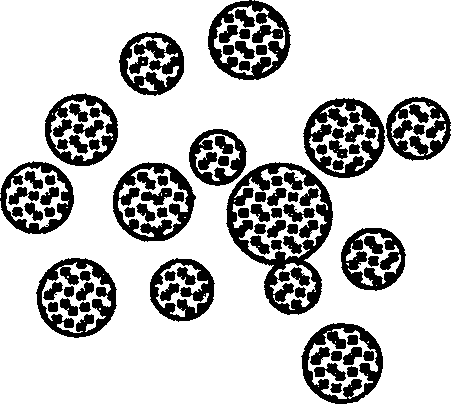
[0027] exist figure 2 In the experimental example shown, the W1 / O phase was slowly and uniformly injected into the high-speed stirring W2 with a syringe. After the final clathrate, the sample was taken and observed through a microscope. It can be observed that the W1 / O / W2 phase is an opaque spherical microstructure. The diameter of the microsphere is obviously larger than that of the W1 / O phase.
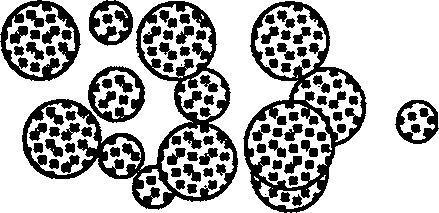
[0028] exist image 3 In another experimental example shown, W1 / O / W2 microspheres are aged by a rotary evaporator to evaporate methylene chloride, the collected microspheres are added with excipients, and a specific lyophilization curve is applied in a lyophilizer Microscopic observation of th...
experiment example 2
[0029] Experimental Example 2: Release of Lactide-Glycolide Copolymer
[0030] See Figure 4 , Figure 5
Description of drawings:
[0031] figure 1 It is a structural diagram of the W1 / O phase of the present invention.
[0032] figure 2 It is the structure diagram of W1 / O / W2 phase.
[0033] image 3 Structural diagram of microspheres.
[0034] Figure 4 Release curve of PLGA50 / 50
[0035] Figure 5 Release curve of PLGA65 / 35
Embodiment 1
[0037] Thymopentin 7.5g Lactide-glycolide copolymer (PLGA) 40g
[0038] Gelatin 0.75g D-mannitol 0.75g
[0039] Thymopentin was fully dissolved in water to make water phase W1, lactide-glycolide copolymer (PLGA) was fully dissolved in dichloromethane to make oil phase O; polyvinyl alcohol (PVA) was fully dissolved in water to make Form water phase W2; add oil phase O to water phase W1, emulsify to form W1 / O phase, then slowly inject into water phase W2 at a uniform speed to form W1 / O / W2 phase, evaporate through rotary evaporation Organic solvent dichloromethane, collect the microspheres, use a specific freeze-drying curve to freeze-dry, and finally make 1000 thymopentin sustained-release microspheres for injection, with a particle size of 1-100um. One injection per month, 4 to 5 mg per injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com