Fmoc-strategy solid-phase synthesis method of thymopentin
A solid-phase synthesis and thymus technology, which is applied in the preparation of polypeptide drugs and the field of solid-phase synthesis of thymopentin, can solve the problems of increased transportation and storage costs, reduced scientific research convenience, and inconvenience for pharmaceutical companies, and achieves easy transportation and storage. The effect of storage, chemical stability and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) The connection between the first amino acid and the resin:
[0050] ①Weigh the resin: Take 4g of Wang resin (with a degree of substitution of 1.02mmol / g) in the 80ml reactor No. 1 (accurately weigh its weight before the reaction), the total substitution of Wang resin is the degree of substitution multiplied by the mass, that is 4.08 mmol;
[0051] ② Bonding reaction between resin and amino acid: accurately weigh 4.08mmol (1874.90 mg) of Fmoc-Tyr(tBu)-OH in a 50ml centrifuge tube, add 30ml of DCM (dichloromethane) to dissolve it and add it to No. 1 reactor , To make the resin swell uniformly, the swelling time is about 30min; at the same time, add 4.08mmol (841.826 mg) DCC (dicyclohexylcarbodiimide), 0.408mmol (49.845mg) DMAP (4-two) into another 50ml centrifuge tube. Methylaminopyridine) and 10 mL of DCM, dissolve by mediation, quickly pour it into the No. 1 reactor, mediate uniformly, and oscillate with the swollen resin for about 4 hours to obtain Fmoc-Tyr(tBu)-Wang; ...
Embodiment 2
[0079] (1) The connection between the first amino acid and the resin:
[0080] ①Weigh the resin: take 4g of Wang resin (with a degree of substitution of 1.02mmol / g) in the reactor (accurately weigh its weight before the reaction), the total substitution of Wang resin is the degree of substitution multiplied by the mass, which is 4.08 mmol;
[0081] ② Bonding reaction between resin and amino acid: accurately weigh 3.627~4.488mmol Fmoc-Tyr(tBu)-OH and place it in a centrifuge tube, add 30ml DCM mediation to dissolve and add it to the reactor to make the resin swell evenly, the swelling time is about 30min ; At the same time, add 3.627~4.488mmol DCC, 0.3627~0.4488mmol DMAP and 10mL DCM in another centrifuge tube. After mediation dissolve, quickly pour into the reactor, mediation uniformly, and swell the resin evenly, shake reaction for about 4h to obtain Fmoc- Tyr(tBu)-Wang.
[0082] (2) Head of excess resin
[0083] ①Head capping reaction: Wash all the resin in the reactor with DMF unt...
Embodiment 3
[0103] In the present invention, DMF solutions of piperazine concentrations of 0.1 mol / L, 0.25 mol / L, 0.75 mol / L, and 1 mol / L piperazine are selected as the deprotection agent, and the solvent of the piperazine solution is also selected as NMP (N- Methylpyrrolidone), THF (tetrahydrofuran), DMSO (dimethyl sulfoxide) and other dipolar aprotic solvents, referring to the operating method of Example 2, the corresponding dipeptide, tripeptide, tetrapeptide intermediates can also be prepared , And thymopentin.
[0104]
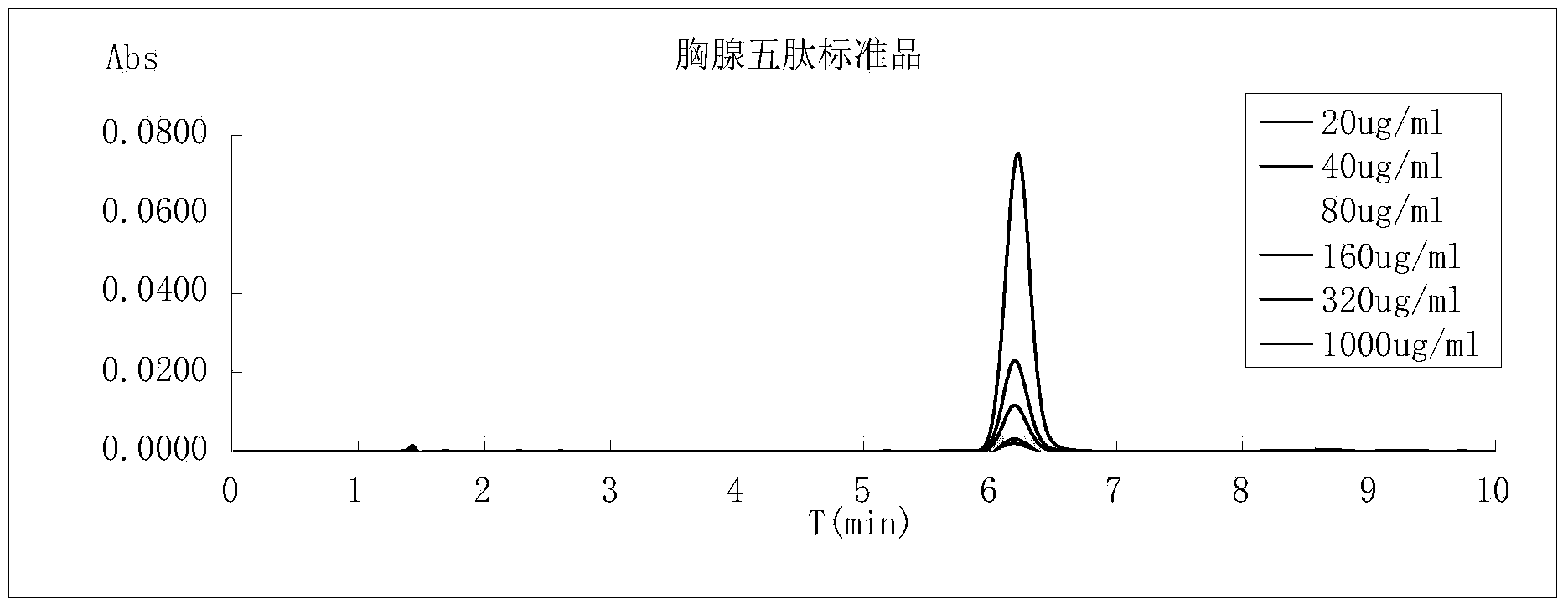
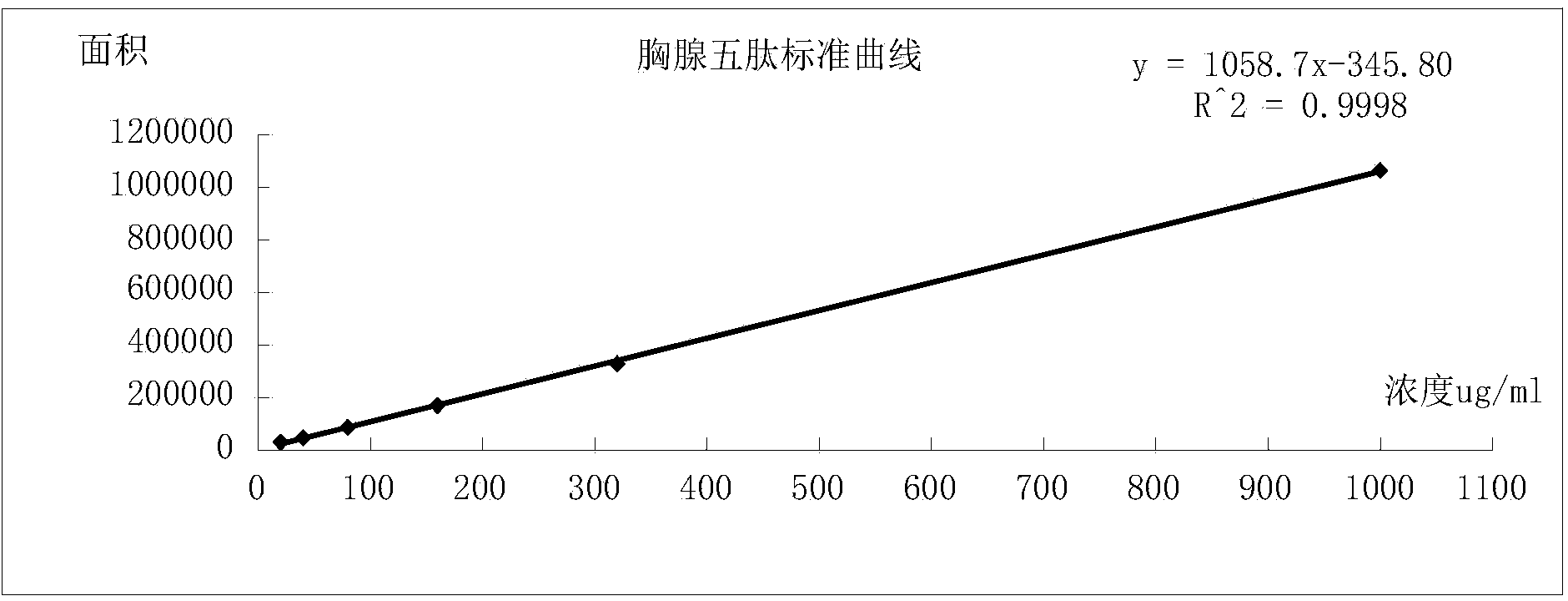
[0105] In the following, the polypeptide intermediates and thymopentin prepared in the examples are tested and compared with the corresponding products prepared using the traditional deprotecting agent piperidine.
[0106] 1. Determination of Wang resin loading
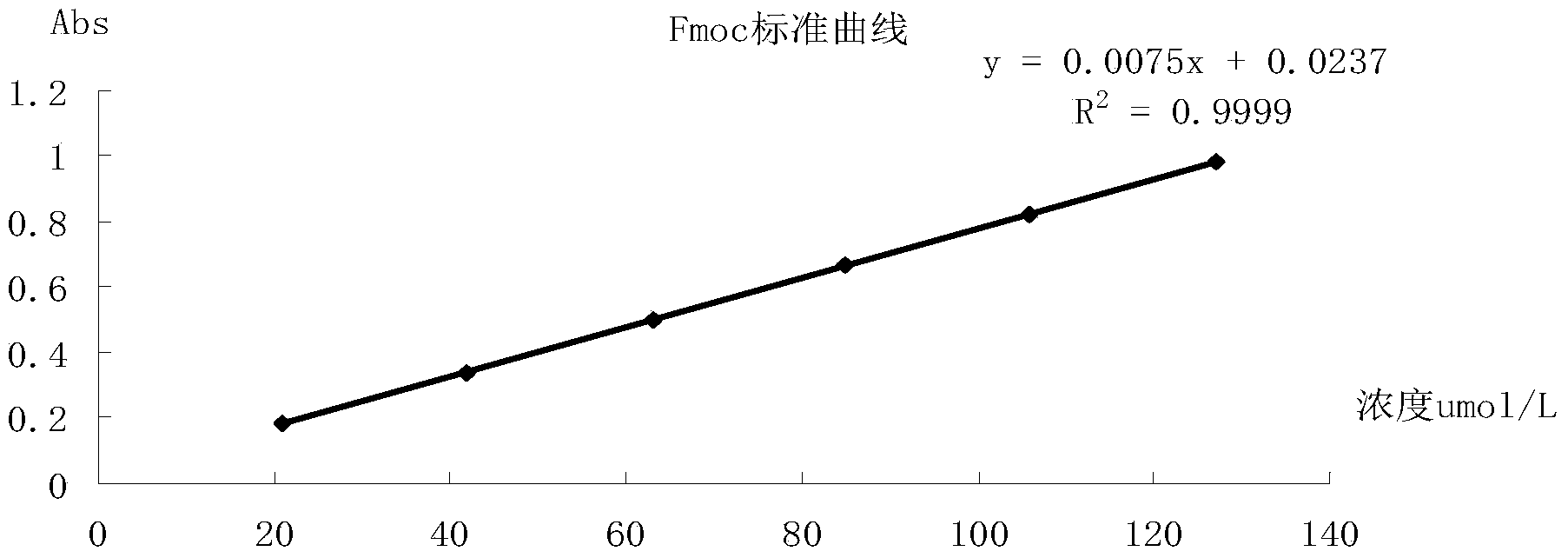
[0107] (1) Make Fmoc standard curve
[0108] ① Using Fmoc-Ala as the standard substance, accurately weigh 39.5 mg of Fmoc-Ala-OH, treat with 1ml 20% pip / NMP for 20 minutes, and dilute 0.1ml of it with NMP to 10ml. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com