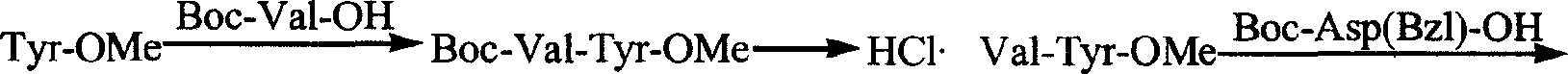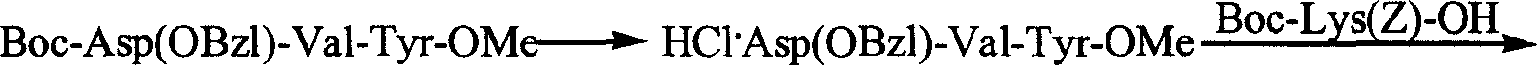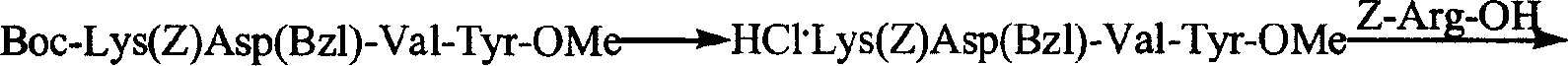Process for preparing thymopentin
A pentapeptide and thymus technology, which is applied in the field of preparation of Jing-Lai-Tian-Val-Tyropentapeptide, can solve the problems of limited product quantity, waste, high risk and the like, and achieves low synthesis cost, prevention of side reactions, and high price. cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0060] The present invention will be further illustrated by non-limiting examples below.
[0061] Herein, the abbreviations for the reagents used are:
[0062] DCC: Dicyclohexylcarbodiimide
[0063] HOBt: 1-Hydroxybenzotriazole
[0064] NMM: N-methylmorpholine
[0065] THF: Tetrahydrofuran
Embodiment
[0067] 1. Preparation of Boc-Val-Tyr-OMe
[0068] 3.52g (15.2mmol) of HCl·Tyr-OMe was dissolved in 20ml of anhydrous tetrahydrofuran, the resulting solution was adjusted to pH 7-8 with N-methylmorpholine, cooled to 0°C for later use.
[0069] 3.29g (15.2mmol) Boc-L-Val-OH, 2.15g (16.0mmol) HOBt were dissolved in anhydrous tetrahydrofuran, cooled to 0°C, slowly added 3.29g (16.0mmol) DCC, stirred at 0°C for 5 minutes. Then mix with the above solution, stir at 0°C for 2 hours, maintain pH 8-9 with N-methylmorpholine, stir at room temperature for 10 hours, chloroform / methanol / acetic acid (20 / 1 / 0.4) shows that the raw material point disappears. The reaction mixture was filtered, the filtrate was concentrated under reduced pressure, the residue was repeatedly ground with petroleum ether, and the viscous substance obtained was dissolved in 250ml ethyl acetate, and the 3 Aqueous solution (30ml×5), saturated NaCl aqueous solution (30ml×1), 5% KHSO 4 Aqueous solution (30ml×5), washed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com