Abuse resistant drug formulation
a drug formulation and drug technology, applied in the direction of biocide, microcapsules, drug compositions, etc., can solve the problems of significant side effects, inability to meet the needs of patients, so as to reduce the amount of drug abus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
FIGS. 1&2 of 069
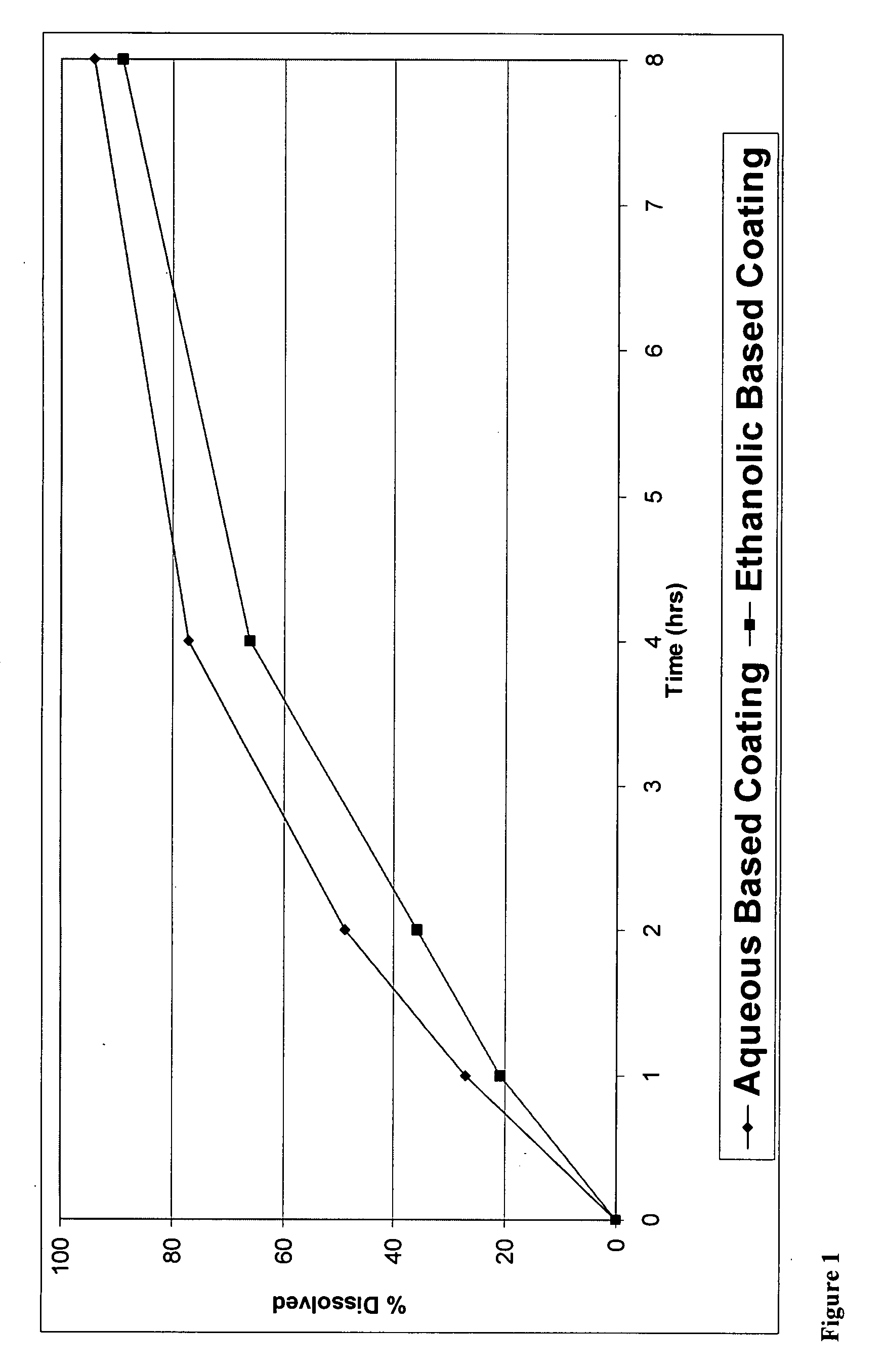
[0176]The present invention can be illustrated by producing CR coated particles with wet granules as API particles.
TABLE 1Granules FormulationComponent% (w / w)Oxycodone Hydrochloride27.8Hydroxypropyl methylcellulose 84446.3Ethylcellulose25.9
TABLE 2Coated Granules FormulationComponent% (w / w)Oxycodone Granules50.0Ethylcellulose33.3Magnesium Stearate16.7
[0177]Granules were manufactured in a high shear granulator where oxycodone hydrochloride, HPMC 844 and 71% of the total amount of ethylcellulose were dry mixed for 2 minutes. Then, a 10% hydro-ethanolic (30:70) solution of ethylcellulose was slowly added while maintaining the granulator impeller and chopper speeds at pre-selected values to provide enough shear for granule formation and growth. Solution addition was continued until the aforementioned percentage of ethylcellulose was realized. The granules were subsequently dried in a fluid bed to a level that renders them suitable for milling. The granules were then mille...
example 2
FIGS. 1&2 of 069
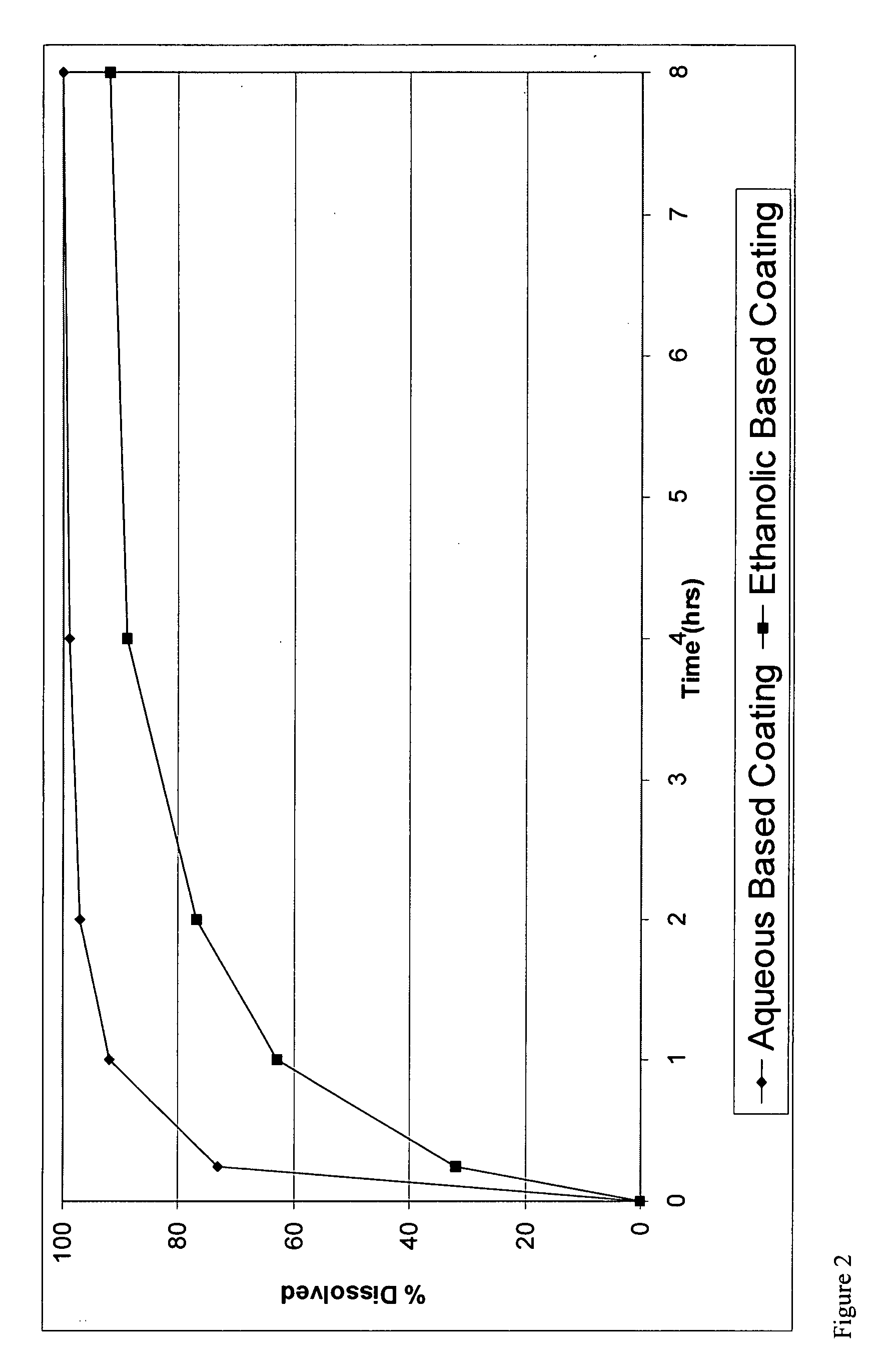
[0180]The methods of making coated particles, described above in Example 1 were employed again except the formulation was coated with the aqueous EC dispersion.
TABLE 3Granules FormulationComponent% (w / w)Oxycodone Hydrochloride27.8Hydroxypropyl methylcellulose 84446.3Ethylcellulose25.9
TABLE 4Coated Granules FormulationComponent% (w / w)Oxycodone Granules50.0Surelease ® (25% Solid)50.0
The coating used was a SURELEASE aqueous dispersion (Commercial Aqueous dispersion of EC from Colorcon Manufacturer Lot #1N509251) The dissolution results of uncrushed (FIG. 1) and crushed (FIG. 2) particles from the aqueous coating are shown in plots using diamonds indicating the measured data points.
example 3
From 069
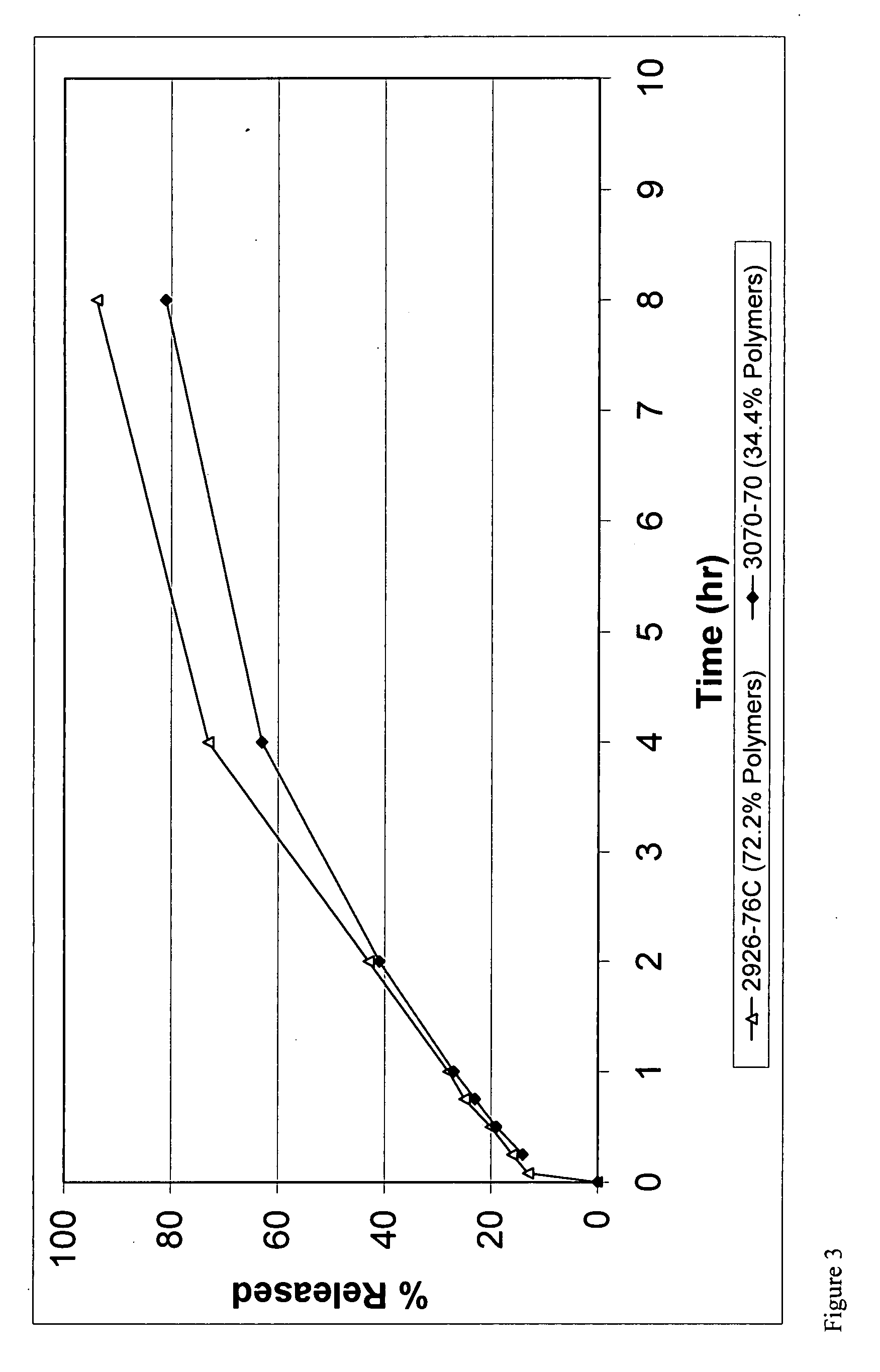
[0181]
TABLE 5Granules FormulationComponent% (w / w)Oxycodone Hydrochloride46.1Hydroxypropyl methylcellulose 84436.9Ethylcellulose17.0
TABLE 6Coated Granules FormulationComponent% (w / w)Oxycodone Granules50.0Ethylcellulose33.3Magnesium Stearate16.6
The same manufacturing method as used in Example 1 can be used except only 54% of EC is dry mixed with other ingredients instead of 71%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com