Medicinal composition for treatment of chronic hepatitis c
a technology for chronic hepatitis c and composition, which is applied in the direction of drug compositions, antibody medical ingredients, peptide/protein ingredients, etc., can solve the problems of not being able to clarify whether hcv invades a cell or only adheres to its surface, and the infection of chronic hcv becomes a serious problem, so as to prevent side effects of hepatic cirrhosis and enhance immune function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-1
Induction of MICA / B Expression by Cytokine
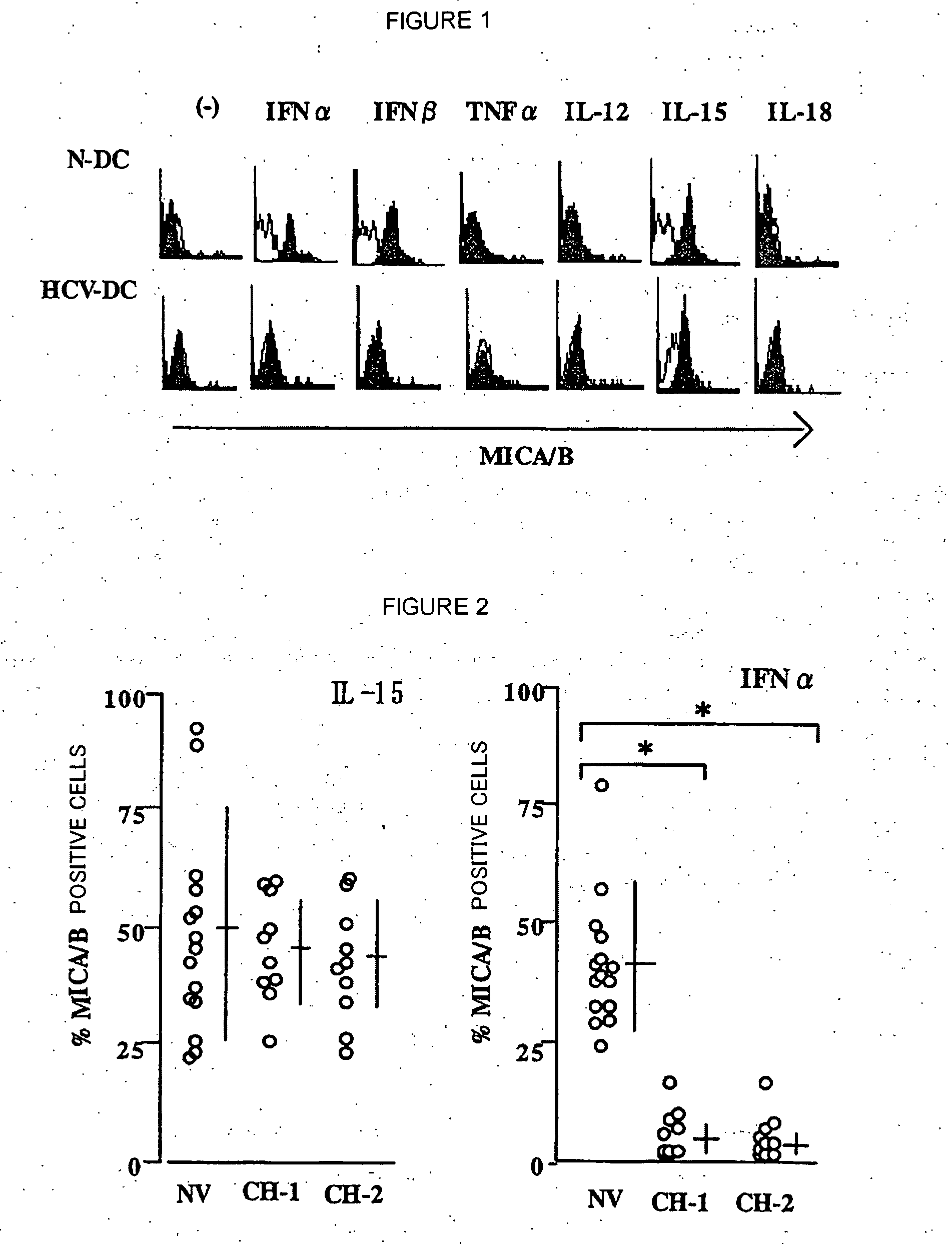
[0073] Whether MICA / B is induced by various cytokines or not was investigated in DCs derived from healthy donors and HCV-infected patients having or not having ALT abnormality. Monocyte-derived DCs from healthy donors (N-DC) and HCV-infected patients (HCV-DC) were prepared, and at day 6 of culturing, stimulated for 24 hours with IFNa (1000 U / mL), IFNβ (1000 U / mL), TNFa (10 ng / mL), IL-12 (10 ng / mL), IL-15 (50 ng / mL) and IL-18 (20 ng / mL). Then, MICA / B expression was analyzed by flow cytometry. The results are shown in FIG. 1.
[0074] DCs from normal donors (N-DC) responded to IFNa or IFNβ to express MICA / B, however, DCs from HCV-infected patients (HCV-DC) did not express MICA / B in any cases. IL-15 could apparently induce MICA / B, in N-DC and HCV-DC. In contrast, TNFa, IL-12 or IL-18 did not induce MICA / B expression.
example 1-2
RT-PCR Analysis of MICA / B mRNA
[0075] Total RNAs were isolated from DCs not stimulated, DCs stimulated with IL-15 (50 ng / mL) and DCs stimulated with IFNa (1000 U / mL), respectively, and expression of MICA mRNA and expression of MICB mRNA were analyzed by RT-PCR. Consequently, results corresponding to the above-mentioned results of flow cytometry were obtained.
example 1-3
Difference of MICA / B Expression Depending on Presence or Absence of ALT Abnormality
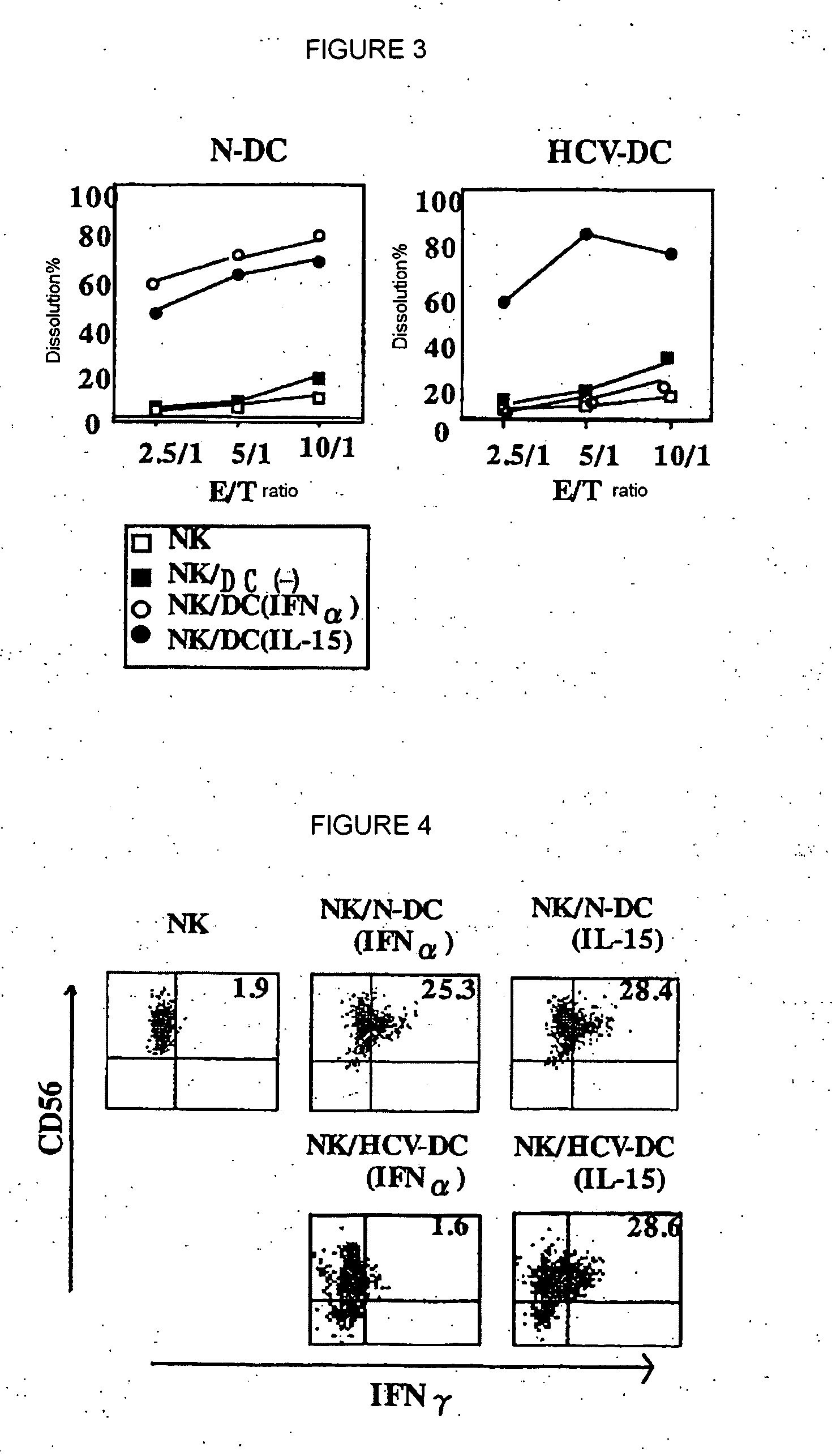
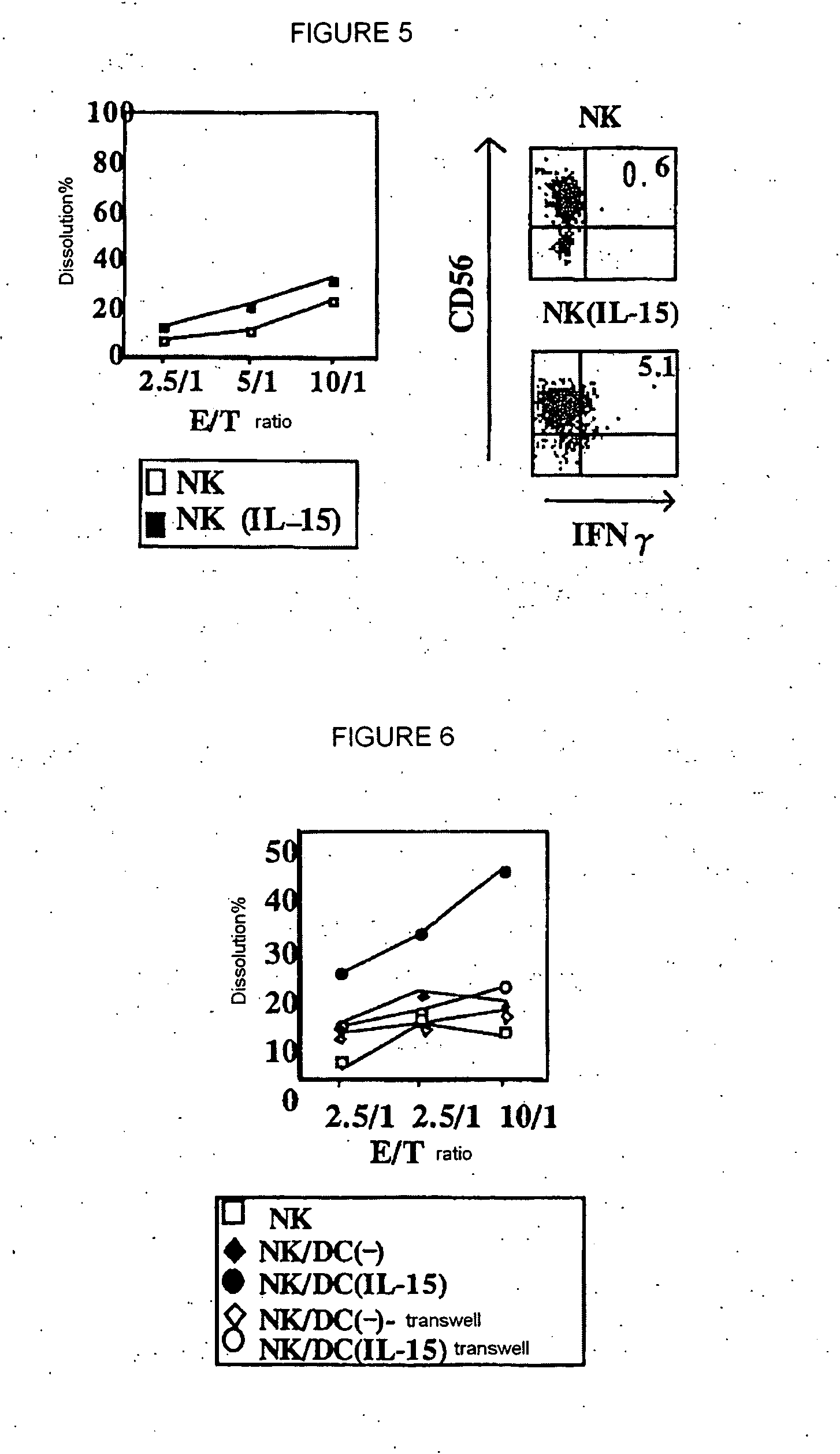
[0076] MICA / B expression was compared among healthy donors (n=15)(HV), chronic HCV-infected patients showing abnormal ALT (n=10)(CH-1) and chronic HCV-infected patients having persistent normal ALT level (n=10)(CH-2). MICA / B expression of DCs stimulated with IFNa or IL-15 was checked by flow cytometry, and represented as percent of positive cells. The results are shown in FIG. 2. In the drawing, a horizontal bar represents an average, a vertical bar represents SD, and * represents p<0.01. HCV-DC, when stimulated with IFNa, did not express MICA / B irrespective of the condition of ALT abnormality. This suggests that no MICA / B expression in response to IFNa or IFNβ in HCV-DC is not caused by chronic inflammation of liver.
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com