Inhibitors of DNA immunostimulatory sequence activity
a technology of immunostimulatory sequence and inhibitor, which is applied in the field of immunostimulatory sequence inhibitors in dna, can solve the problems of preventing the host from obtaining the potential benefits of affecting the clinical practice of both gene therapy and gene immunization, and achieving transient gene expression in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example ii
Assay to Confirm IIS-ON Inhibitory Activity As Measured by a Reduction in INF-.gamma. Secretion
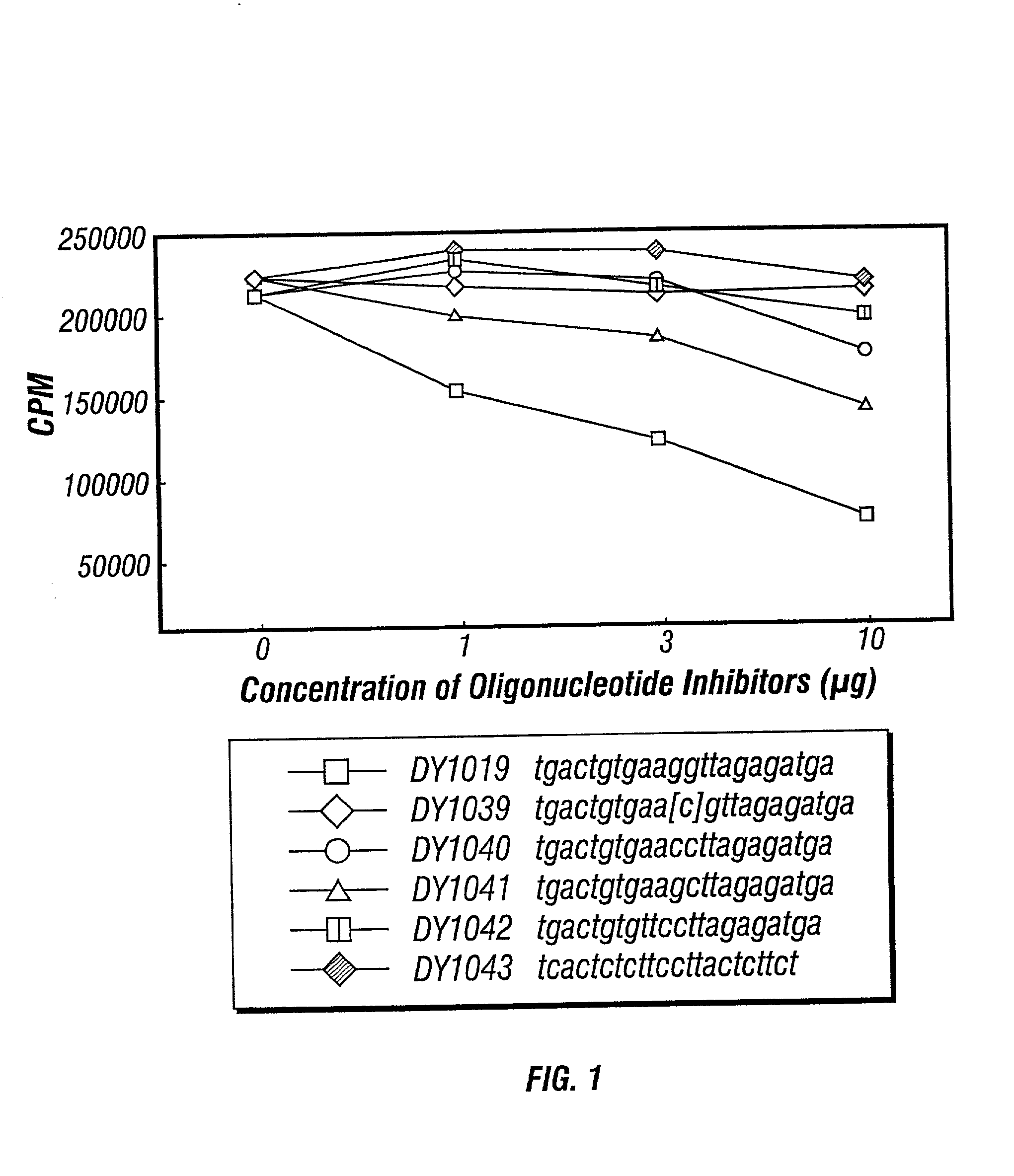
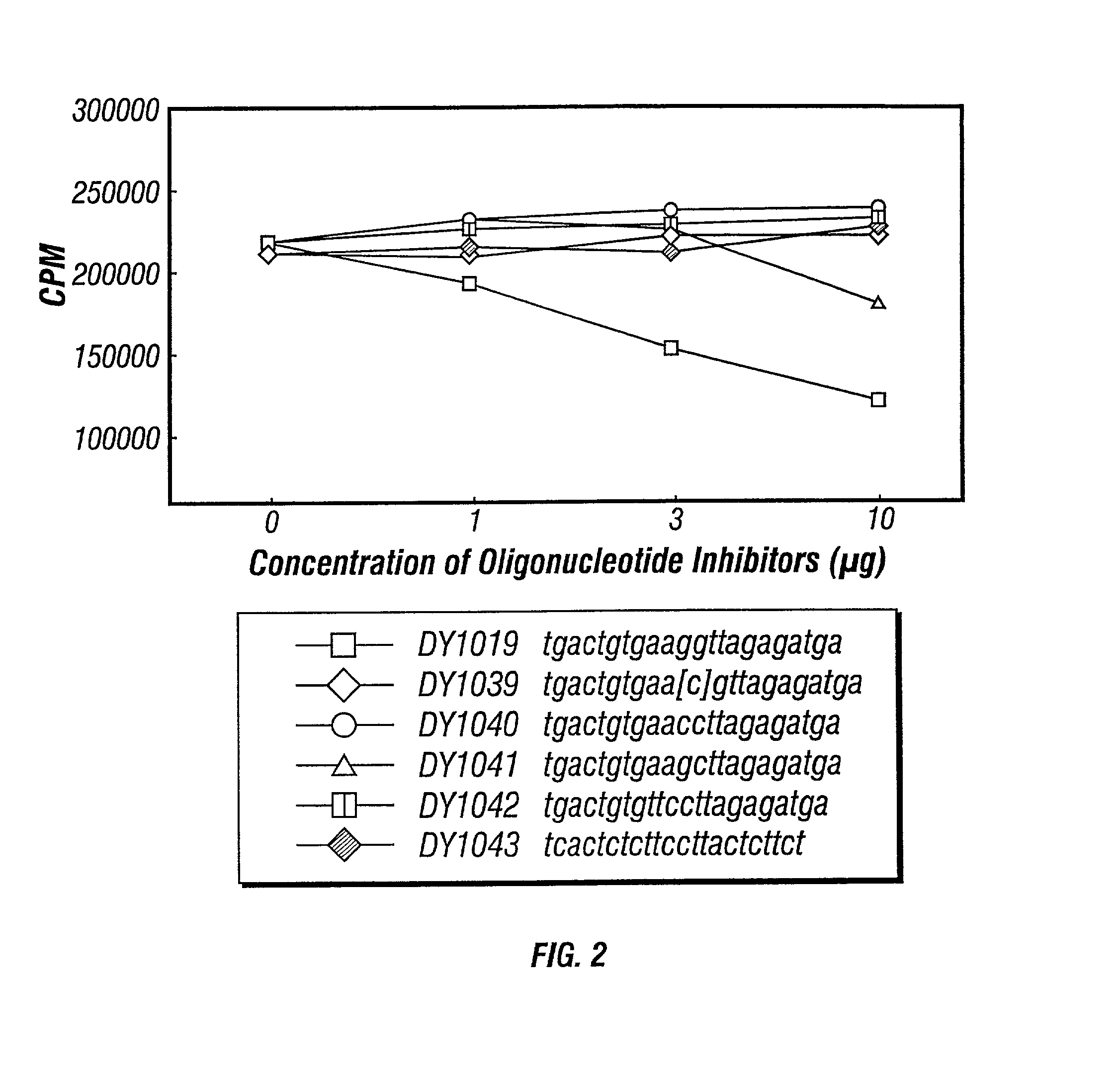
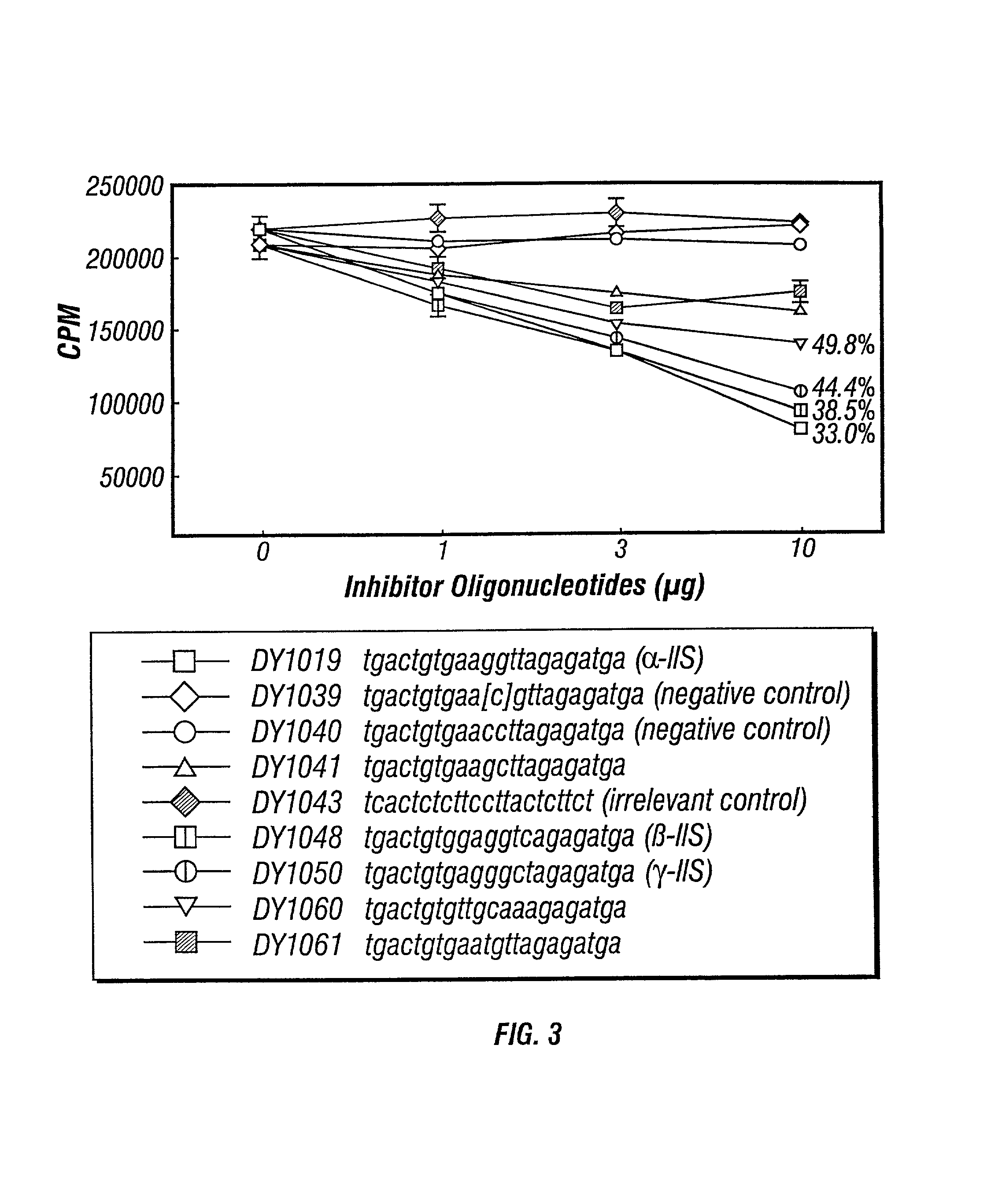
[0113] Groups of mice were immunized as described in Example I, sacrificied and their splenocytes harvested. Supernatants of harvested splenocytes was incubated with 1 .mu.g / ml of DY1018 ISS-ODN in saline as described in Example I. Within 4 hours, the supernatants were incubated with various concentrations of IIS-ON or a control. DY1039 (an ISS with the cytosine methylated), DY1040 and DY1043 (the latter with CC dinucleotides in place of the CG dinucleotide of DY1018) served as controls (all oligonucleotide sequences are set forth in the legend to the FIGURES and in the Description of Drawings). To confirm the location of potential competition with DY1018, all of the oligonucleotides were identical to DY1018 except for the hexamer regions identified and DY1043 (an irrelevant sequence control).
[0114] IFN-.gamma. levels were measured pre- and post-IIS-ODN contact. Any observable changes in I...
example iii
IIS-ODN Boosting of Th2 Type Immune Responses to Antigen
[0116] Groups of four Balb / c mice were co-immunized with 10 .mu.g .beta.-galactosidase antigen and 50 .mu.g (in 50 .mu.l normal saline) of IIS-ODN DY1019 (identified in the Figure as .beta.-gal / M-ODN), the ISS-ODN composition .beta.-gal / ISS-ODN (5'-AATTCAACGTTCGC-3'), the .beta.-gal antigen and pKISS-3 (a plasmid having three copies of the AACGTT ISS-ODN hexamer in the backbone), the .beta.-gal antigen and pKISS-0 (a control plasmid having no copies of the AACGTT ISS-ODN hexamer in the backbone), or saline alone. Th2 responses in each group of mice were measured by ELISA as a function of IgE levels obtained post-boosting. As shown in FIG. 5, potent Th2-type responses (above 1000 CPM) were obtained only in the mice which received saline (approximately 1200 CPM at 1 week post-boosting) and .beta.-gal / M-ODN (approximately 1750 CPM at 1 week post-boosting).
[0117] Further, high levels of IgG2a antibodies and low levels of IgG1 antib...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com