Vaccine against streptococcus pneumoniae capsular polysaccharides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
third embodiment
[0055] In a third embodiment there is provided a vaccine composition comprising a pneumococcal polysaccharide antigen and a Th1 adjuvant.
[0056] Streptococcus pneumoniae Polysaccharide Antigens of the Invention
[0057] Typically the Streptococcus pneumoniae vaccine of the present invention will comprise polysaccharide antigens (preferably conjugated), wherein the polysaccharides are derived from at least four serotypes of pneumococcus. Preferably the four serotypes include 6B, 14, 19F and 23F. More preferably, at least 7 serotypes are included in the composition, for example those derived from serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. More preferably still, at least II serotypes are included in the composition, for example the composition in one embodiment includes capsular polysaccharides derived from serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F (preferably conjugated). In a preferred embodiment of the invention at least 13 polysaccharide antigens (preferably conjugated) a...
example 1
[0149] S.pneumoniae Capsular Polysaccharide:
[0150] The 11-valent candidate vaccine includes the capsular polysaccharides serotypes 1, 3, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F which were made essentially as described in EP 72513. Each polysaccharide is activated and derivatised using CDAP chemistry (WO 95 / 08348) and conjugated to the protein carrier. All the polysaccharides are conjugated in their native form, except for the serotype 3 (which was size-reduced to decrease its viscosity).
[0151] Protein Carrier:
[0152] The protein carrier selected is the recombinant protein D (PD) from Non typeable Haemophilus influenzae, expressed in E. coli.
[0153] Expression of Protein D
[0154] Haemophilus influenzae Protein D
[0155] Genetic Construction for Protein D Expression
[0156] Starting Materials
[0157] The Protein D Encoding DNA
[0158] Protein D is highly conserved among H. influenzae of all serotypes and non-typeable strains. The vector pHIC348 containing the DNA sequence encoding the entire prot...
example 2
[0204] Study of the Effect of Advanced Adjuvants on the Immunogenicity of the 11-Valent Pneumococcal PS-PD Conjugate Vaccine in Infant Rats
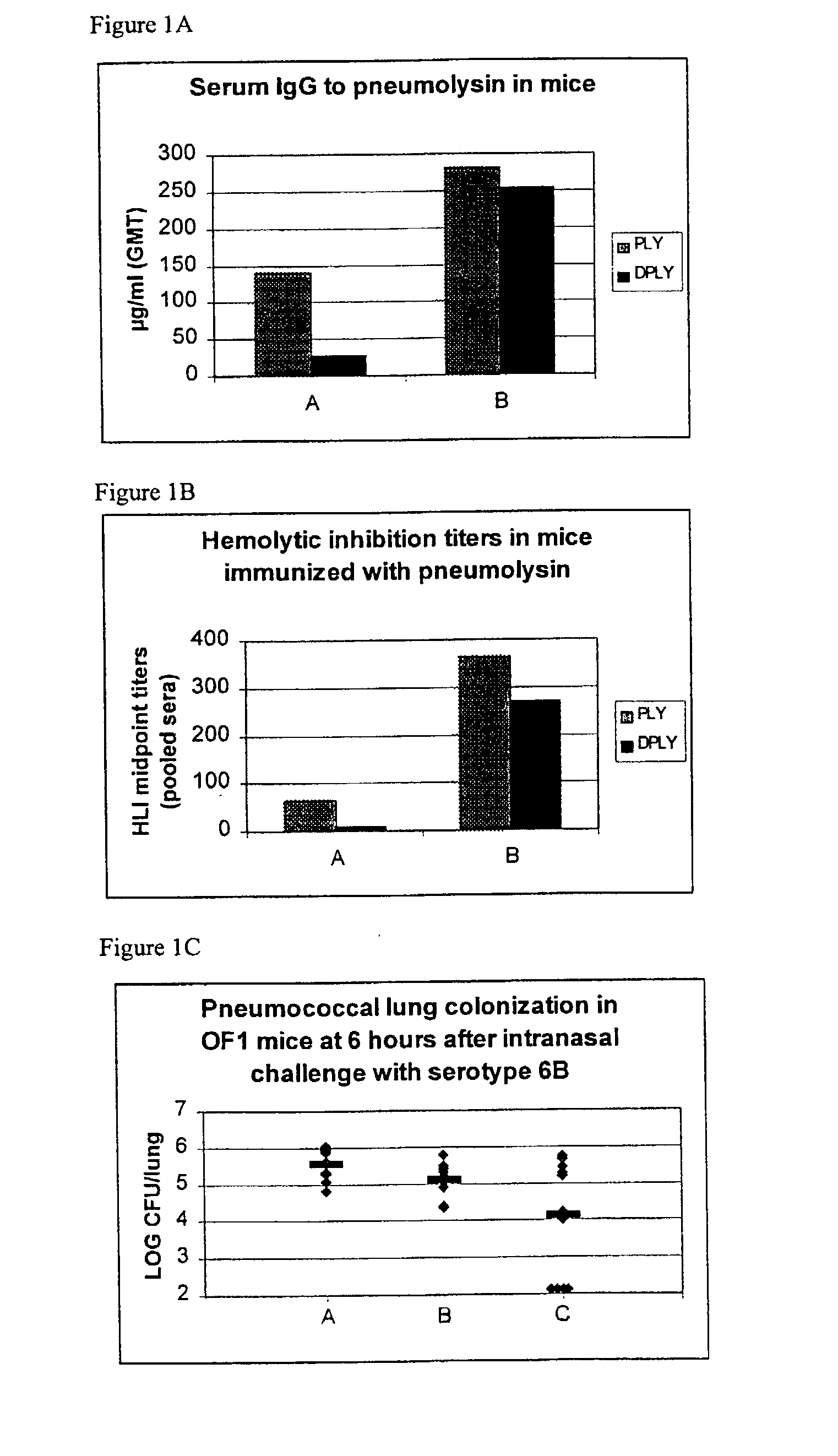
[0205] Infant rats were immunised with 11 valent pneumococcal PS-PD conjugate vaccine at a dosage of 0.1 .mu.g each polysaccharide (made according to the method of Example 1), and using the following adjuvant formulations: none, AlPO.sub.4, 3D-MPL, 3D-MPL on AlPO.sub.4.
[0206] The formulation with only 3D-MPL was statistically (and surprisingly) more immunogenic (greatest GMC IgG) than for the other formulations for 5 out of 11 antigens. This was true both at high and low concentrations of 3D-MPL.
[0207] Opsonophagocytosis confirmed the GMC results.
[0208] Materials and Methods
[0209] Immunisation Protocol
[0210] Infant OFA rats were randomised to different mothers and were 7 days old when they received the first immunisation. They received 2 additional immunisations 14 and 28 days later. A bleed was performed on day 56 (28 days post III). All vaccine...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Force | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com