Novel medicinal composition and preparation method
A technology of composition and medicine, which is applied in the field of new pharmaceutical composition and preparation, can solve the problems of weak inductive ability, achieve the effects of reducing dosage, low treatment cost and increasing anti-lung cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
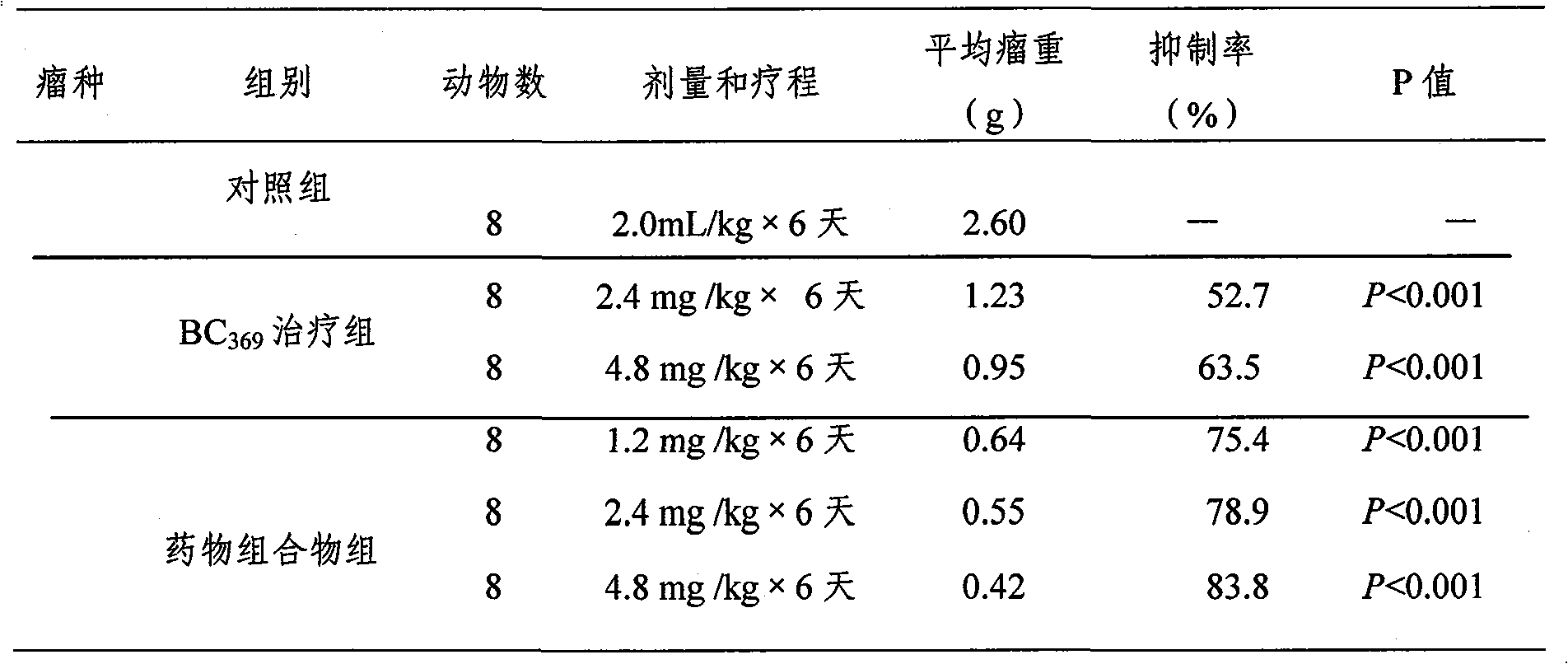
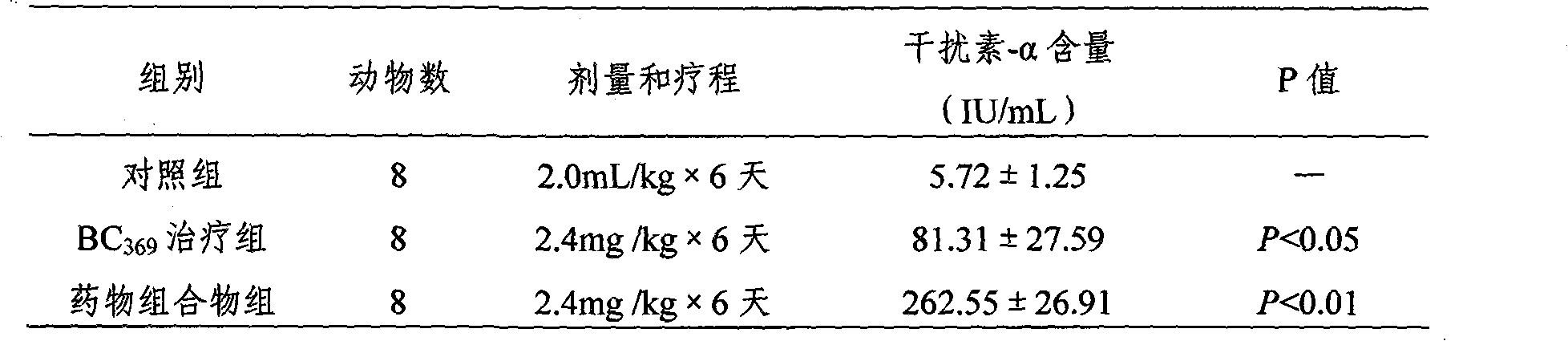
Image
Examples
specific Embodiment 1
[0029] (1) Selection and cultivation of chicken Newcastle disease virus
[0030] Chicken Newcastle disease virus is selected, cultured in the allantoic fluid of 10-12 day old live chicken embryos at 37-38° C. for 48-72 hours, and then the allantoic fluid containing the virus is collected.
[0031] The titer was determined by the hemagglutination method, and then the titer of the chicken Newcastle disease virus allantoic fluid determined was adjusted to 1:640.
[0032] The impurities, protein, contaminated bacteria and part of urate in the allantoic fluid of chicken embryos are removed through a closed double-layer poplin bag and a 0.22 μm microporous membrane filter.
[0033] (2) Preparation of preparation components
[0034] A: Glycoprotein G 1 0.72 g Glycoprotein G 2 0.36 grams
[0035] Dissolve in 160 ml of 0.075NHCL solution in sequence, and place in a water bath for half an hour.
[0036] B: Biotin 1.0mg
[0037] Dissolve in 7.5 ml of deionized water first, add 0.1 ...
specific Embodiment 2
[0069] (1) Selection and cultivation of chicken Newcastle disease virus
[0070] Chicken Newcastle disease virus is selected, cultured in the allantoic fluid of 10-12 day old live chicken embryos at 37-38° C. for 48-72 hours, and then the allantoic fluid containing the virus is collected.
[0071] The titer was determined by the hemagglutination method, and then the titer of the chicken Newcastle disease virus allantoic fluid determined was adjusted to 1:640.
[0072] The impurities, protein, contaminated bacteria and part of urate in the allantoic fluid of chicken embryos are removed through a closed double-layer poplin bag and a 0.22 μm microporous membrane filter.
[0073] (2) Preparation of preparation components
[0074] A: Glycoprotein G 1 0.72 g Glycoprotein G 2 0.36 grams
[0075] Dissolve in 160 ml of 0.075NHCL solution in sequence, and place in a water bath for half an hour.
[0076] B: Biotin 1.0 mg
[0077] Dissolve in 7.5 ml of deionized water first, add 0.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com