Process for producing physiologically active protein using genetically modified silkworm
a technology of genetically modified silkworm and protein, which is applied in the field of process for producing physiologically active protein using genetically modified silkworm, can solve the problems of difficult to extract and purify the difficulty of purifying the target protein from the body fluid of the silkworm, so as to achieve the effect of reducing the amount of time and labor required for inoculation of recombinant virus, reducing the amount of time and labor required,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Bombyx mori Genomic DNA
[0088]Fifth instar third day silkworms were dissected to remove posterior silk gland tissue. After washing with 1×SSC, 200 μl of DNA extraction buffer (50 mM Tris-HCl (pH 8.0), 1 mM EDTA (pH 8.0), 100 mM NaCl) were added. After adding Proteinase K (final concentration: 20 μg / ml) and adequately grinding up the tissue with a grinder, 350 μl of DNA extraction buffer and 60 μl of 10% SDS were added followed by incubating for 2 hours at 50° C. After adding 500 μl of Tris-HCl-saturated phenol (pH 8.0) and mixing for 10 minutes, the supernatant was recovered by centrifuging for 5 minutes at 4° C. and 10,000 rpm. After adding an equal volume of phenol / chloroform / isoamyl alcohol (25:24:1) to the supernatant and mixing, the resulting mixture was centrifuged. Phenol / chloroform / isoamyl alcohol was again added followed by centrifuging and recovery of the supernatant. After adding an equal volume of chloroform / isoamyl alcohol (24:1) and mixing, the mixture wa...
example 2
Gene Preparation
[0089]The genes used were acquired by PCR by producing primers for the sequences on both ends using known sequences and using suitable DNA sources for the templates. Restriction sites were added to the ends of the primers for the subsequent gene construction procedure.
[0090]Feline interferon-ω gene (base numbers 9-593 of GenBank registration no. S62636) was acquired by PCR using two types of primers consisting of primer 3 (SEQ. ID No. 3) and primer 4 (SEQ. ID No. 4) and using baculovirus rBNV100 encoding feline interferon-ω gene for the template. rBNV100 can be produced by, for example, cutting out FeIFN gene from a plasmid extracted from E. coli(pFeIFN1) (Patent Microorganism Depository No. 1633), coupling to a silkworm cloning vector (T. Horiuchi, et al., Agric. Biol. Chem., 51, 1573-1580, 1987), and co-transfecting silkworm established cells with the recombinant plasmid produced and silkworm nuclear polyhedrosis virus DNA.
[0091]Sericin-1 gene promoter (base number...
example 3
Production of Plasmids for Gene Insertion
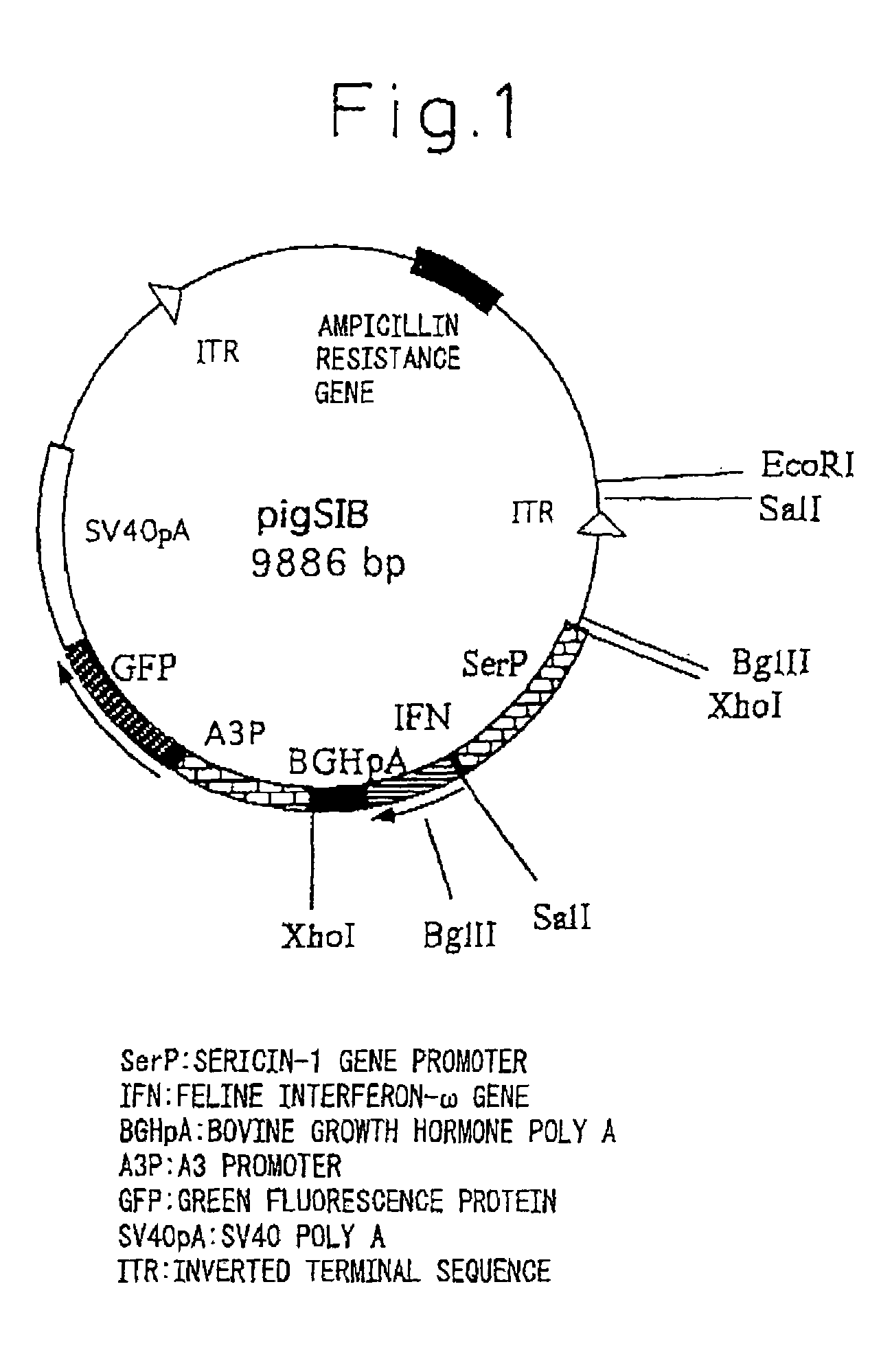
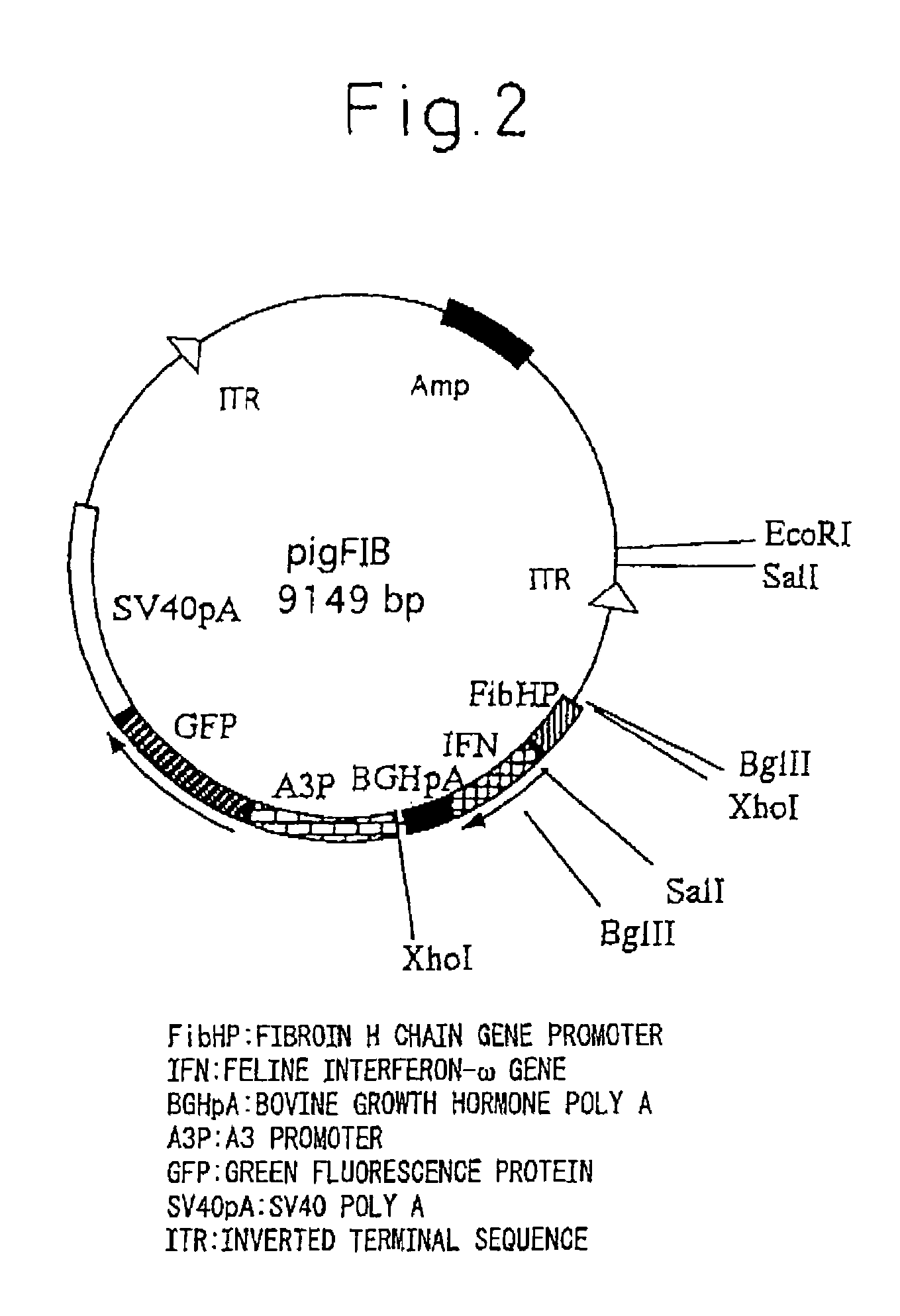
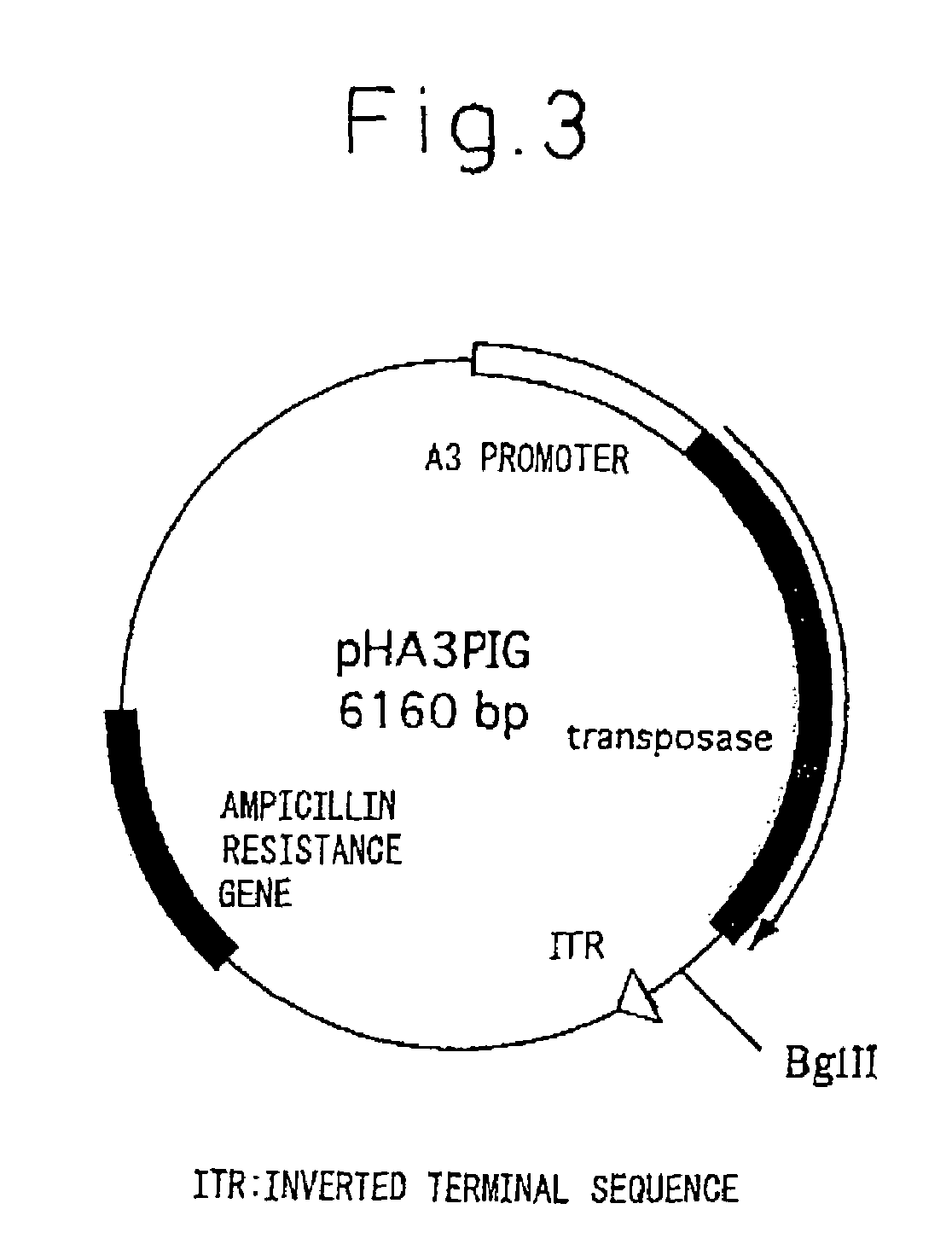
[0094]pigA3GFP (Nature Biotechnology 18, 81-84, 2000) was used for the plasmid for gene insertion. Namely, vector pigA3GFP is a vector in which after removing a region encoding transposase from plasmid p3E1.2 disclosed in U.S. Pat. No. 6,218,185, an A3 promoter (base numbers 1764-2595 of GenBank registration no. U49854), GFP originating in pEGFP-N1 vector (Clontech) and poly A addition sequence originating in SV40 (base numbers 659-2578 of GenBank registration no. U55762) are inserted into that portion (Nature Biotechnology 18, 81-84, 2000). The expression unit of feline interferon-ω gene was inserted at the XhoI site upstream from the A3 promoter. The expression units of the inserted genes consisted of a sericin-1 gene promoter-feline interferon-ω-bovine growth hormone poly A addition sequence (SEQ. ID No. 1), or a fibroin H chain gene promoter-feline interferon-ω-bovine growth hormone poly A addition sequence (SEQ. ID No. 2). The following ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com