Targeted vectors for cancer immunotherapy
a technology of immunotherapy and targeted vectors, applied in the field of cancer, can solve the problems of limited clinical development of these protocols, and achieve the effect of high level efficiency of cytokine gene delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of the GM-CSF Retroviral Expression Vector
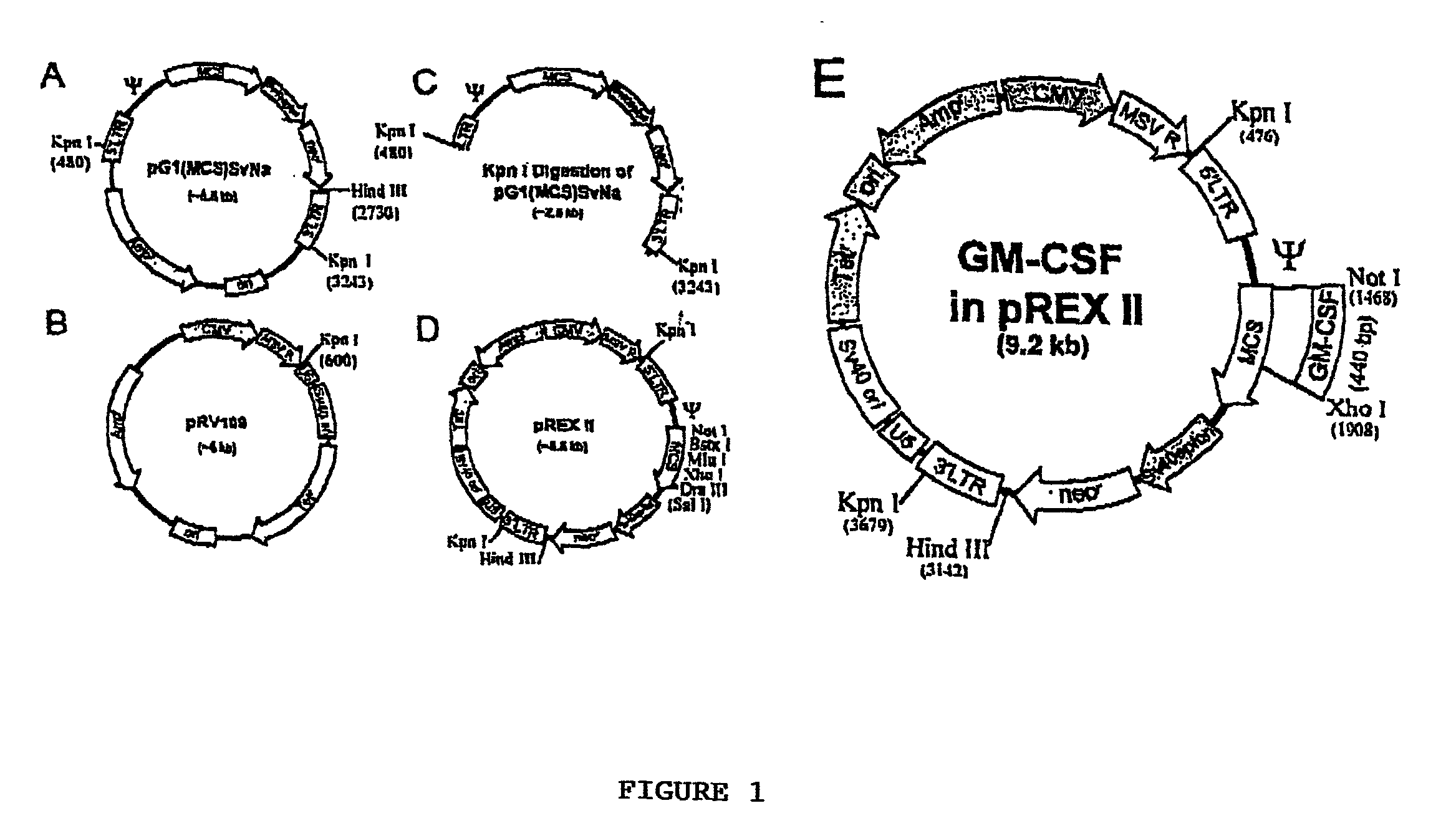
[0046] The retroviral expression vector (pREX 11) was created by engineering a multiple cloning site (MSC) into the G 1 XSvNa vector (Genetic Therapy, Inc.) to produce G 1 (MCS)SvNa (FIG. 1A), which is then subjected to Kpn I digestion followed by fusion of the Kpn I fragment (FIG. 1C) with the linearized pRV 109 vector (FIG. 1B). The resulting pREX II retroviral expression vector (FIG. 1D) is driven by a hybrid CMV / MSV / MLV promoter at the 5' LTR and a standard MLV LTR at the 3' end. Bearing the strong CMV promoter and an SV40 ori, this plasmid is suitable for high titer vector production in 293T cells prepared by transient transfection protocols. (Soneoka et al., 1995). The 0.44 kb cDNA encoding human granulocyte macrophage colony stimulating factor (GM-CSF), GenBank accession number NM 000758, flanked by PCR-derived restriction sites was cloned into the unique Not 1 (5') and Xho 1 (3') cloning sites of the pREX II vector (E).
example 2
Production of Matrix-targeted Retroviral Vectors Bearing a Human GM-CSF Construct
[0047] High titer vectors were generated utilizing a transient three plasmid co-transfection system (Soneoka et al., 1995) in which the packaging components gag-pol, a chimeric MLV-based env bearing a von Willebrand factor-derived collagen-binding (matrix-targeting) motif expressed from the CMV promoter, and a retroviral vector bearing were placed on separate plasmids, each containing the SV40 origin of replication. The resulting vectors are referred to as Mx-GM-CSF, CAE-GM-CSF, Mx null, and Mx-nBg to indicate the envelope and gene encoded in each vector. Mx-GM-CSF is a matrix (i.e. collagen)-targeted vector bearing a human GM-CSF construct. CAE-GM-CSF, is a non-targeted vector bearing the wild type MLV 4070A env protein (Morgan et al., 1993). Mx-null is a matrix-targeted vector bearing only the neo gene, and Mx-nBg, is a collagen-matrix-targeted vector bearing a nuclear targeted .beta.-galactosidase ge...
example 3
GM-CSF Production in Transduced NIH3T3 and 293T Cell Cultures
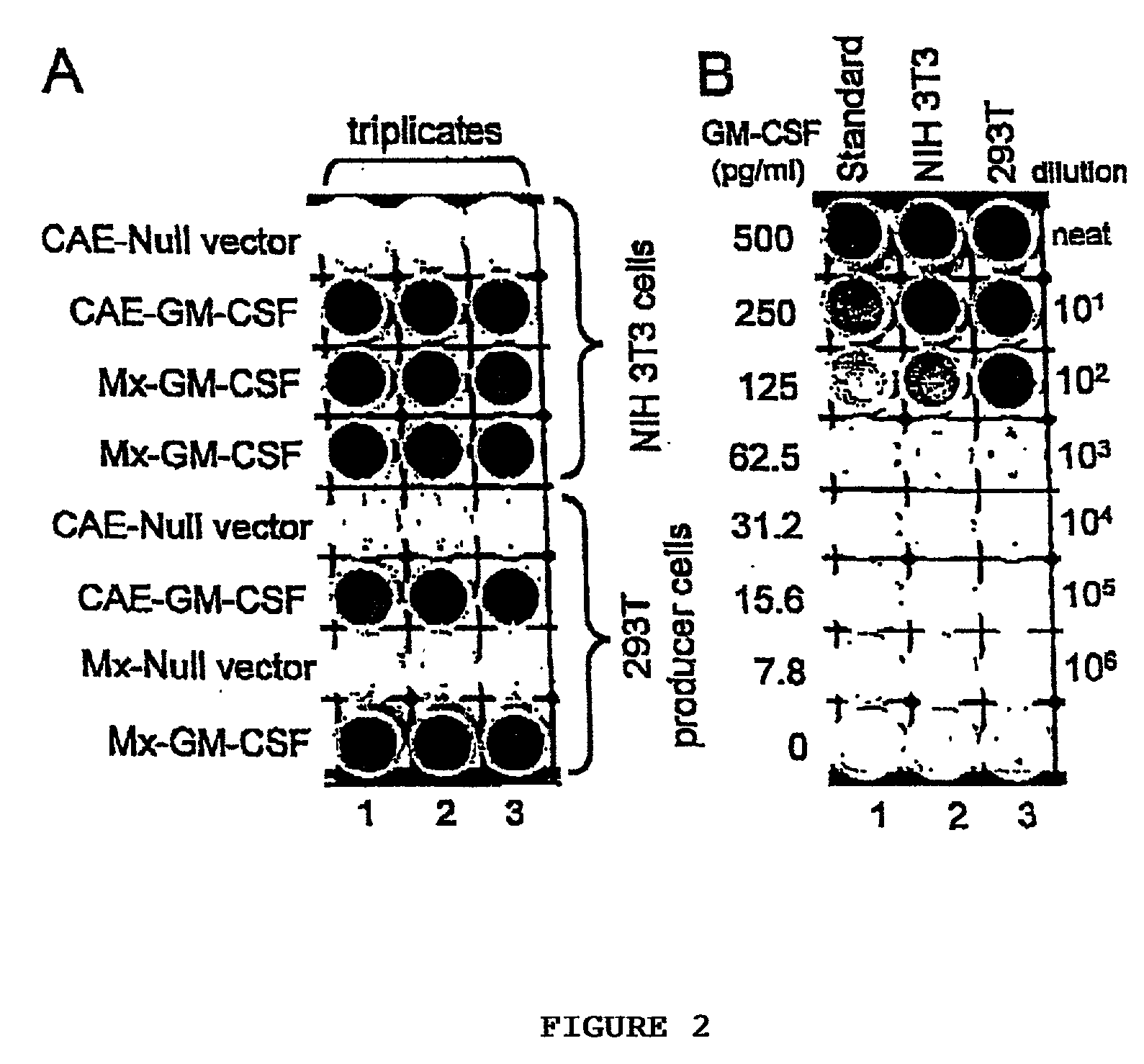
[0048] To assess the production and secretion of GM-CSF, immunohistochemical staining of transduced cells was conducted using a polyclonal goat antibody raised against a peptide, N1 9, mapping at the amino terminus of human GM-CSF (Santa Cruz Biotechnology, Inc.). GM-CSF production was measured in culture medium collected over 3 days in Mx-GM-CSF transduced NIH3T3 and transfected 293T producer cell cultures using commercially available ELISA kits supplied by R&D Systems, Inc.
[0049] Immunoreactive human GM-CSF was noted in 40-50% of transduced NIH3T3 cells and 70-80% of transfected 293T cells (n=4 each group), while GM-CSF production was 32 ng / ml in transduced NIH3T3 cell cultures and 100 ng / ml in transfected 293T cell cultures (FIG. 2).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Surface | aaaaa | aaaaa |
| Coagulation enthalpy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com