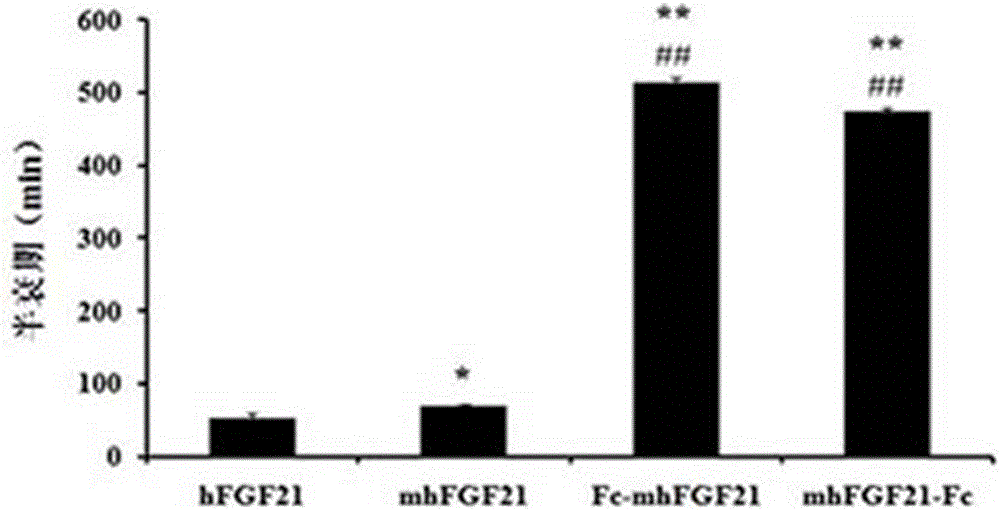
Mutant cytokine fusion protein for treating metabolic diseases
A cytokine and fusion protein technology, applied in metabolic diseases, fibroblast growth factors, cells modified by introducing foreign genetic material, etc., can solve the problems of low efficiency, time-consuming and labor-intensive in mammalian cells, and achieve better hypoglycemic effect. Good, lower blood sugar level, good effect of blood sugar fluctuation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
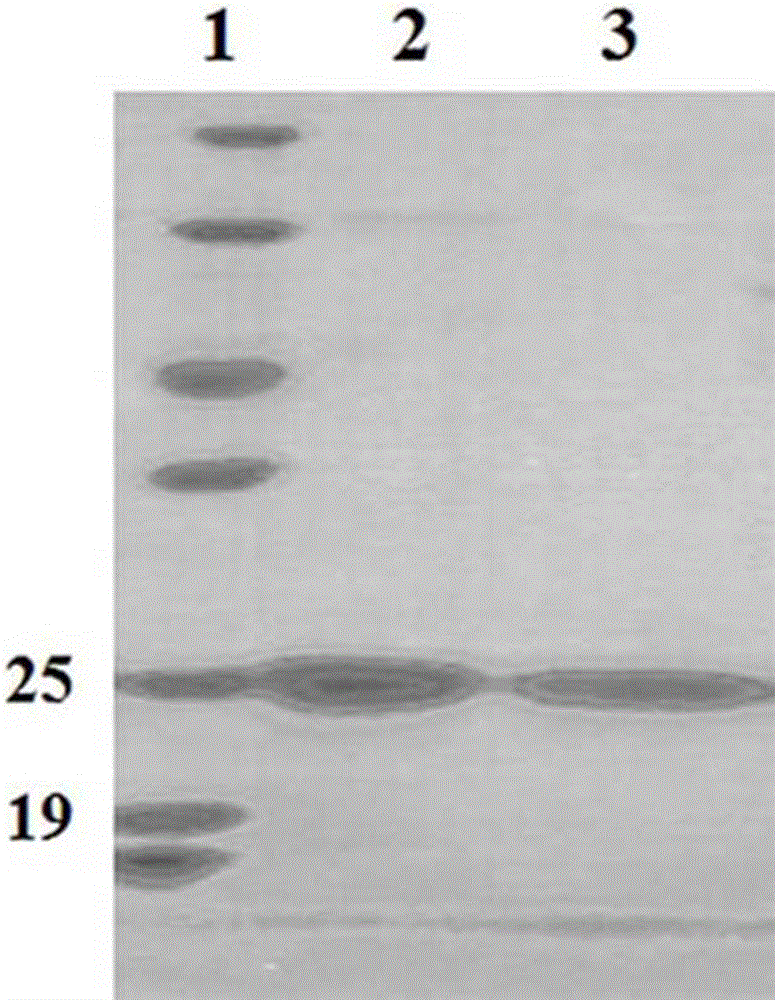
[0035] Example 1 Construction of mhFGF21 gene
[0036] Four primers were designed, and the mhFGF21 protein gene was constructed by overlapping PCR method.
[0037] The 4 primers were designed as follows:
[0038] P1: 5' GGATCCAGCCACCCCATCCCTGACTCCAGTCCTCT 3'
[0039] P2: 5' GGATTCCGGGTGGCTCCGGGGGTGCGGGGGG 3'
[0040]P3: 5' CCAGGCCTGCCCCCCGCAC CCCCGGAGCCACCC 3'
[0041] P4: 5' CGGAATTCTTAGGAAG TGTAGCTGGGGC 3'
[0042] 1. Amplification of the first segment of mhFGF21
[0043] The first segment of the mhFGF21 gene was obtained by using the PCR method with the plasmid containing the hFGF21 gene as a template and P1 and P2 as primers. The amplification system was 25 µl, and the cycle parameters were as follows: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 30 sec, annealing at 50°C for 1 min, extension at 72°C for 1 min, cycle=15, and final extension at 72°C for 10 min. After the amplification, 2 µl of the mixture was taken to observe the amplification results ...
Embodiment 2
[0048] Example 2 Construction of Fc gene
[0049] Referring to molecular cloning methods, total RNA was extracted from human blood with Trizol, and cDNA was reverse-transcribed as a template. Using the Fc of human IgG4 as a template, design the following primers
[0050] P5 5' GCGGAGTCCAAATATGGTCCCCC 3'
[0051] P6 5’ TTAACCCAGAGACAGGGAGA 3’
[0052] The Fc gene of human IgG4 was obtained by using PCR method with cDNA as template and P5 and P6 as primers.
Embodiment 3
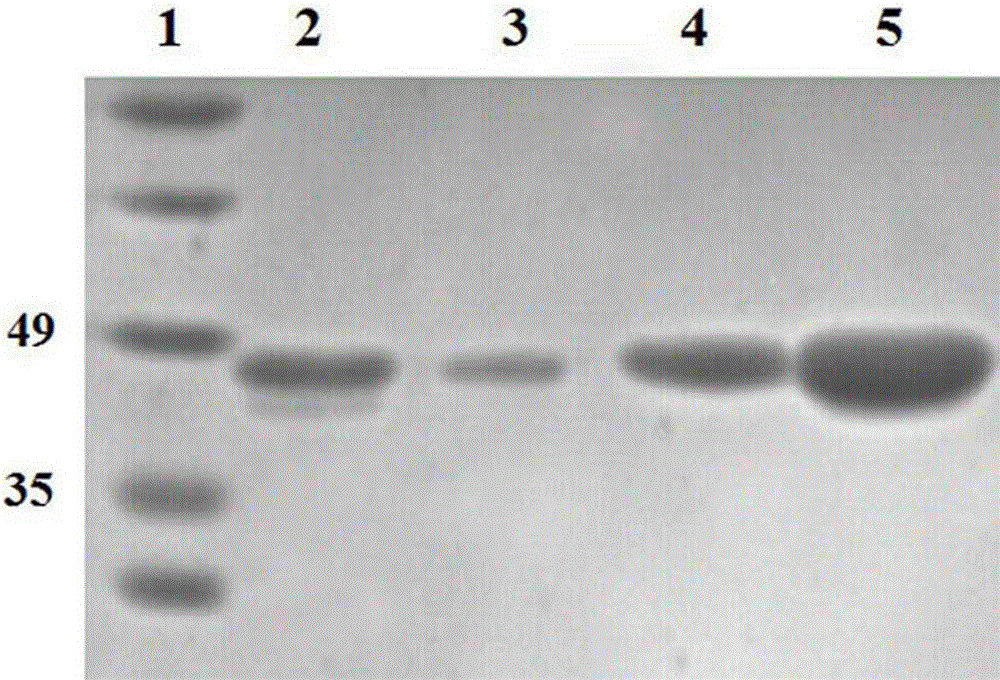
[0053] Example 3 Construction of mhFGF21-Fc gene
[0054] Design 4 primers, use overlapping PCR method to construct mhFGF21-Fc protein gene, use mhFGF21 and Fc (Gly4-Ser) 3 Linker (sequence is 5'GGTGGCGGTGGCTCCGGCGGTGGTGGGTCGGGTGGC GGCGGATCT 3') connection. The 4 primers were designed as follows:
[0055] P7 5 GGATCC AGCCACCCCATCCCTGACTCCAG 3’
[0056] P8 5' CCACCCGACCCACCACCGCCGGAGCCACCGCCACCGGAAGTGTAGCTGGGGCTTC 3'
[0057] P9 5'GGTGGCGGTGGCTCCGGCGGTGGTGGGTCGGGTGGCGGCGGATCTGCGGAGTCCAAAT 3'
[0058] P10 5’CGCGAATTC TTA ACCCAGAGACAGGGAGA 3’
[0059] 1. Amplification of the first segment of mhFGF21-Fc
[0060] The first segment of the mhFGF21-Fc gene was obtained by using the PCR method with the plasmid containing the mhFGF21 gene as a template and P7 and P8 as primers. The amplification system was 25 µl, and the cycle parameters were as follows: pre-denaturation at 95°C for 5 min, denaturation at 95°C for 30 sec, annealing at 48°C for 1 min, extension at 72°C for 1 min, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com