Sesquiterpene compound, preparation method thereof and application thereof in preparation of anti-inflammatory drugs and immunosuppressive drugs
A sesquiterpene and immunosuppressive technology, which is applied in the field of natural medicinal chemistry, can solve the problems that anti-inflammatory and immunosuppressive active ingredients have not yet been discovered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The present invention provides the preparation method of the sesquiterpene compound described in the above scheme, comprising the following steps:
[0028] (1) Extract Xinjiang False Gentiana with an organic solvent to obtain an extract;
[0029] (2) carry out decompression distillation to described extraction liquid, concentrate and obtain medicinal extract;
[0030] (3) after dissolving the extract, perform column chromatography separation and high performance liquid chromatography separation in sequence to obtain the sesquiterpene compound;
[0031] The column chromatography separation includes silica gel column chromatography, MCI column chromatography or reversed-phase C18 column chromatography, Sephadex column chromatography carried out in sequence; phase chromatography.
[0032] The invention uses an organic solvent to extract Xinjiang false gentian to obtain an extract. Before the extraction, the present invention preferably pulverizes the Xinjiang pseudogent...
Embodiment 1
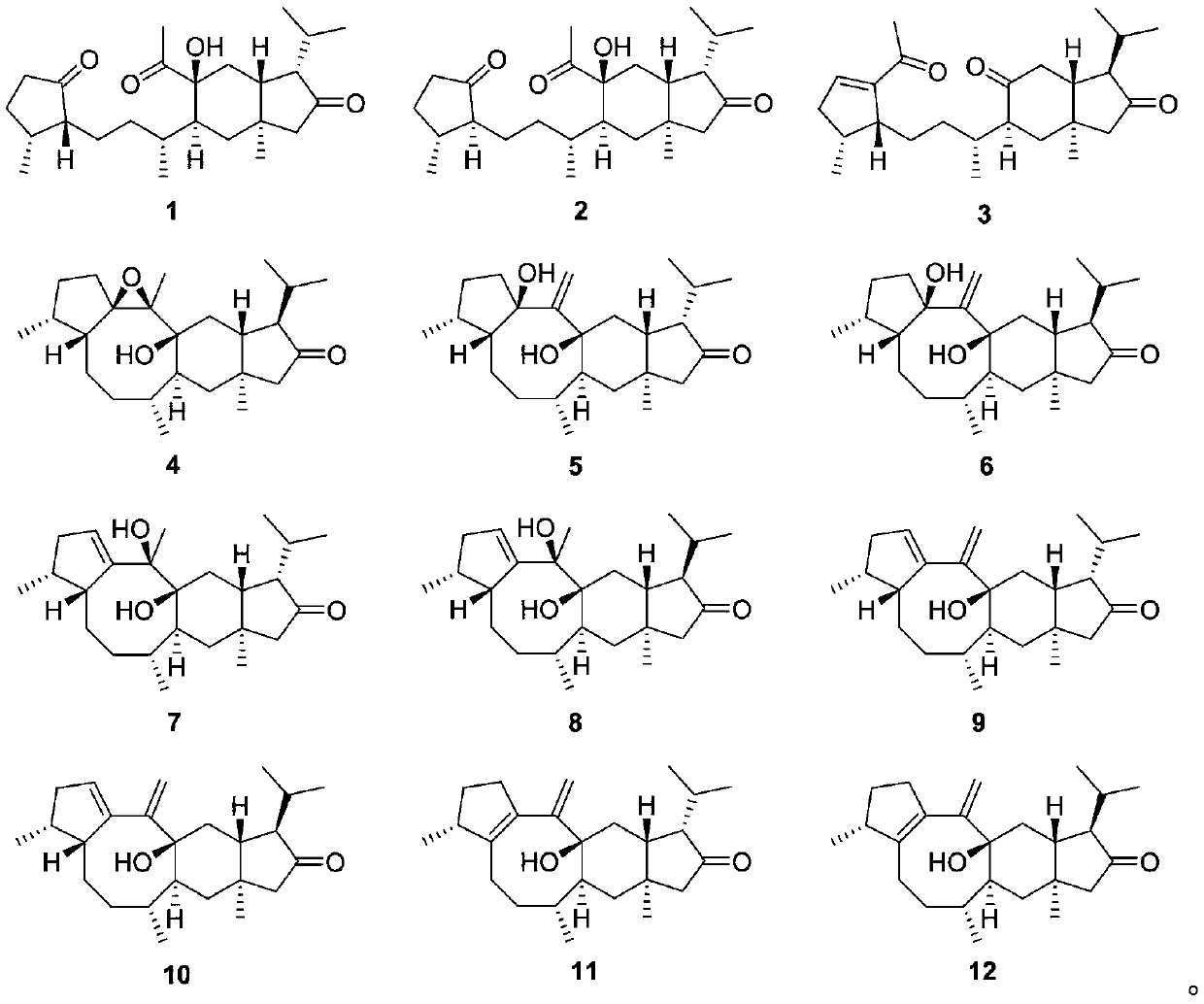
[0043] The extraction and separation of the sesquiterpenoids with structures shown in formula 1 to formula 12, the steps are as follows:
[0044] (1) The whole stem of Xinjiang false gentian collected from Urumqi, Xinjiang, was dried in the shade and crushed to 30 mesh to obtain a 6.0kg sample, soaked and extracted with 40L petroleum ether at room temperature for 5 times, each time for 48h, filtered and combined the extracts, and decompressed After distilling off the solvent, 190 g of total extract was obtained;
[0045] (2) The extract was dissolved in 1.2L of chloroform, mixed with 210g of silica gel (200-300 mesh), placed to dry and ground and sieved, packed with 1.6kg of silica gel (200-300 mesh) for column chromatography, followed by Petroleum ether: chloroform (1:0, 1:1, 0:1, v / v) and chloroform: acetone (9:1, 4:1, 1:1, 0:1) for gradient elution, and the resulting stream Fractions were concentrated under reduced pressure and detected by thin-layer chromatography. After ...
Embodiment 2
[0078] Compounds 1-12 of Example 1 were tested for in vitro immunosuppressive activity:
[0079] CD3, CD28 monoclonal antibody, and IFN-γ detection kits were purchased from BD Bioscience, CCK-8 kits were purchased from Meilun Company, RPMI-1640 medium and fetal bovine serum were purchased from Biological Industries.
[0080] Preparation of samples to be tested: the 12 compounds of Example 1 were respectively dissolved in DMSO to prepare a 20 mM stock solution.
[0081] Specific method: Spleen cells were aseptically isolated from 6-8-week-old female C57BL / 6 mice, and T cells were isolated by nylon hair column method, and then inoculated with T cells (4×10 5 / well) in a 96-well plate (coated with 5 μg / ml CD3 monoclonal antibody), and each group was added with prepared 40, 20, 10, 5, 2.5, 1.25 μM compound and 2 μg / ml CD28 monoclonal antibody, placed in 37℃ / 5%CO 2 cultured in an incubator. After 48 hours, the cell supernatant was collected, and enzyme-linked immunosorbent assay...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com