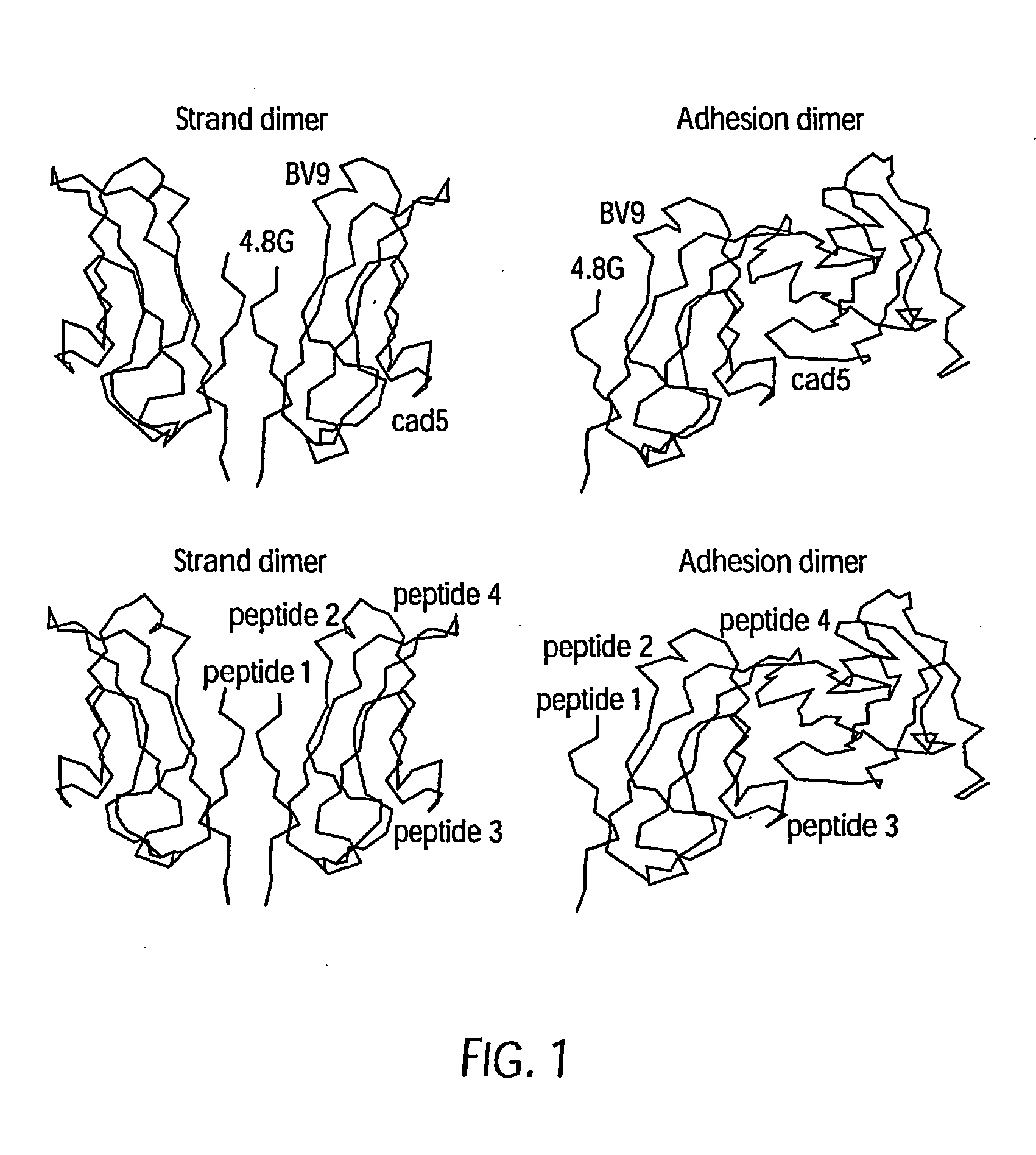
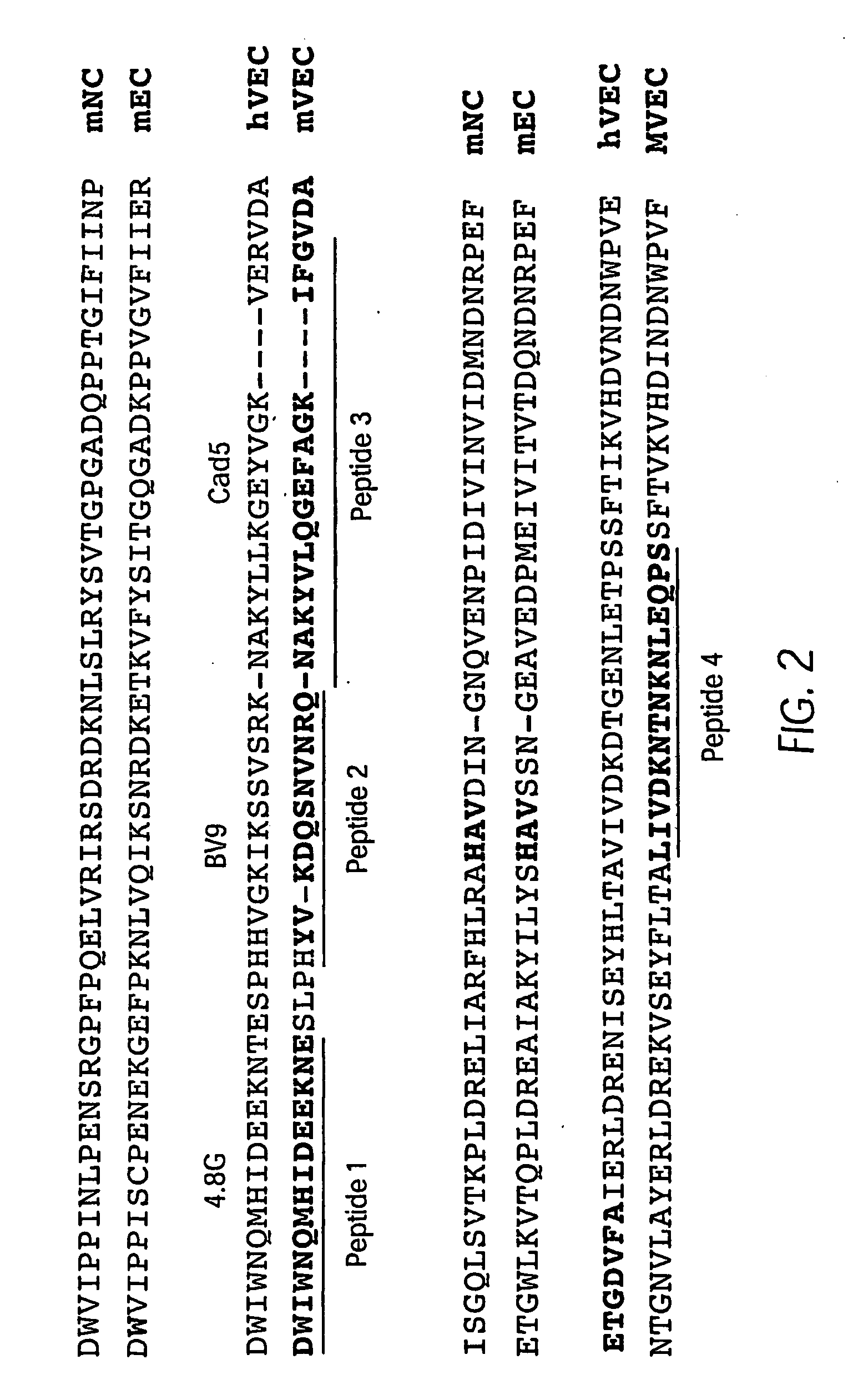
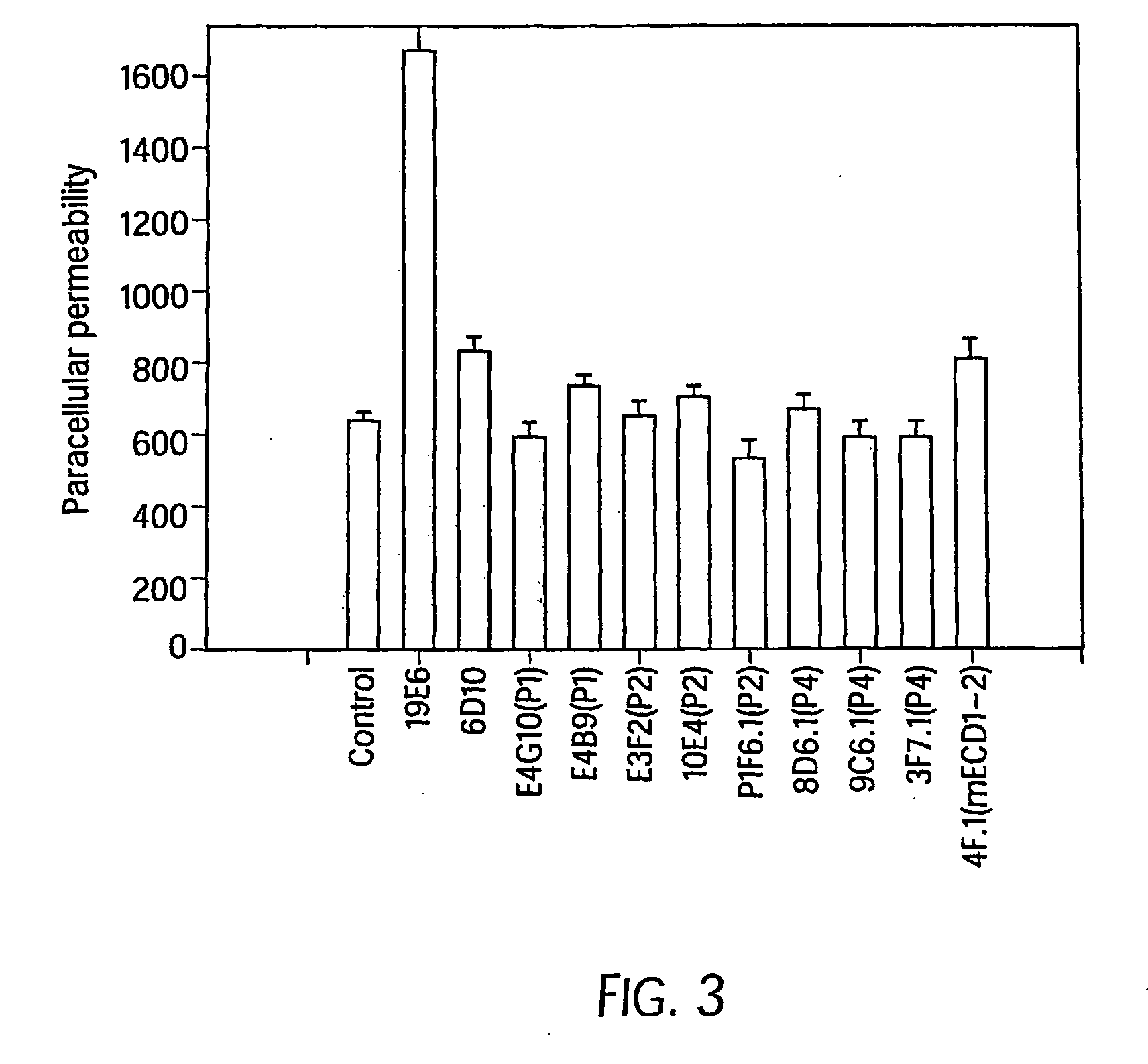
Antibody antagonists of ve-cadherin without adverse effects on vascular permeability
a technology of vecadherin and anti-vecadherin, which is applied in the field of anti-vecadherin anti-antagonizers without adverse effects on vascular permeability, can solve the problems of vascular leak syndrome, hemorrhage and death, and disturbance of normal vasculature integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods
[0078]Monoclonal Antibody Preparation: Lewis rats (6-8 week old females) were injected subcutaneously (s.c.) with 0.1 ml of protein or peptide mixed in Freund's complete adjuvant using a 25-gauge needle. Rats were boosted every 2-3 weeks with antigen and bled via the tail vein every week. After 3 booster immunizations or when sera titers reach maximal levels, mice were sacrificed by CO2 inhalation. Spleens were recovered from sacrificed animals for monoclonal antibody generation by conventional techniques.
[0079]Antibody Screening: Hybridoma supernatants were screened in by an enzyme-linked immunosorbent assay (ELISA) to identify antibodies which bound to VE-cadherin.
[0080]Junction Formation / Ca Switch Assay: The junction formation assay was developed based on a modification of the calcium switch assay (Gumbiner, B., & Simons, K., Cell Biol. 102:457-468 (1986)). Transfectant CHO cells or endothelial cells expressing VE-cadherin are plated onto glass slides and allowed to form a...
example 2
VE-Cadherin Monoclonal Antibodies That Inhibit Adherens Junction Formation Without Disrupting Existing Junctions
[0084]Two groups of Lewis rats were immunized with either a mixture of four KLH-coupled peptides having sequences from the N-terminal domain 1 of murine VE-cadherin (FIG. 2) or with affinity-purified soluble mouse VE-cadherin (smVEC-Ig) which had been expressed in CHO cells. This immunogen encompasses the entire extracellular region of mouse VE-cadherin fused to human Fc chain. The resulting hybridoma clones were tested for production of antibodies with binding activity to VE-cadherin using a conventional ELISA format. This screening identified twenty (20) rat anti-murine VE-cadherin antibodies, 10 from each of the originally immunized groups of rats.
[0085]Several properties of these monoclonal antibodies were examined and the results are summarized in Tables 1 and 2.
[0086]The 20 candidate VE-cadherin antibodies were tested in the “calcium-switch” and “permeability” assays...
example 3
E4B9 Cross Reacts with Human VE-Cadherin
[0087]The murine epitope sequence recognized by antibody E4B9 shares 100% homology with human VE-cadherin, so this antibody was examined to determine if it cross-reacts with human VE-cadherin. Western-blot analysis of several VE-cadherin expressing human and murine cell indicated that E4B9 indeed cross-reacts with human VE-cadherin (FIG. 6). This finding facilitates development of a “humanized” E4B9 antibody and its success in the preclinical development since its anti-tumor activity can be tested extensively in several mouse models.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| paracellular permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com