Brown fat cell compositions and methods
A technology of brown adipose tissue and brown fat, which is applied in the field of stem cell culture and application, and can solve the problem of identifying stem cell populations without brown adipose tissue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
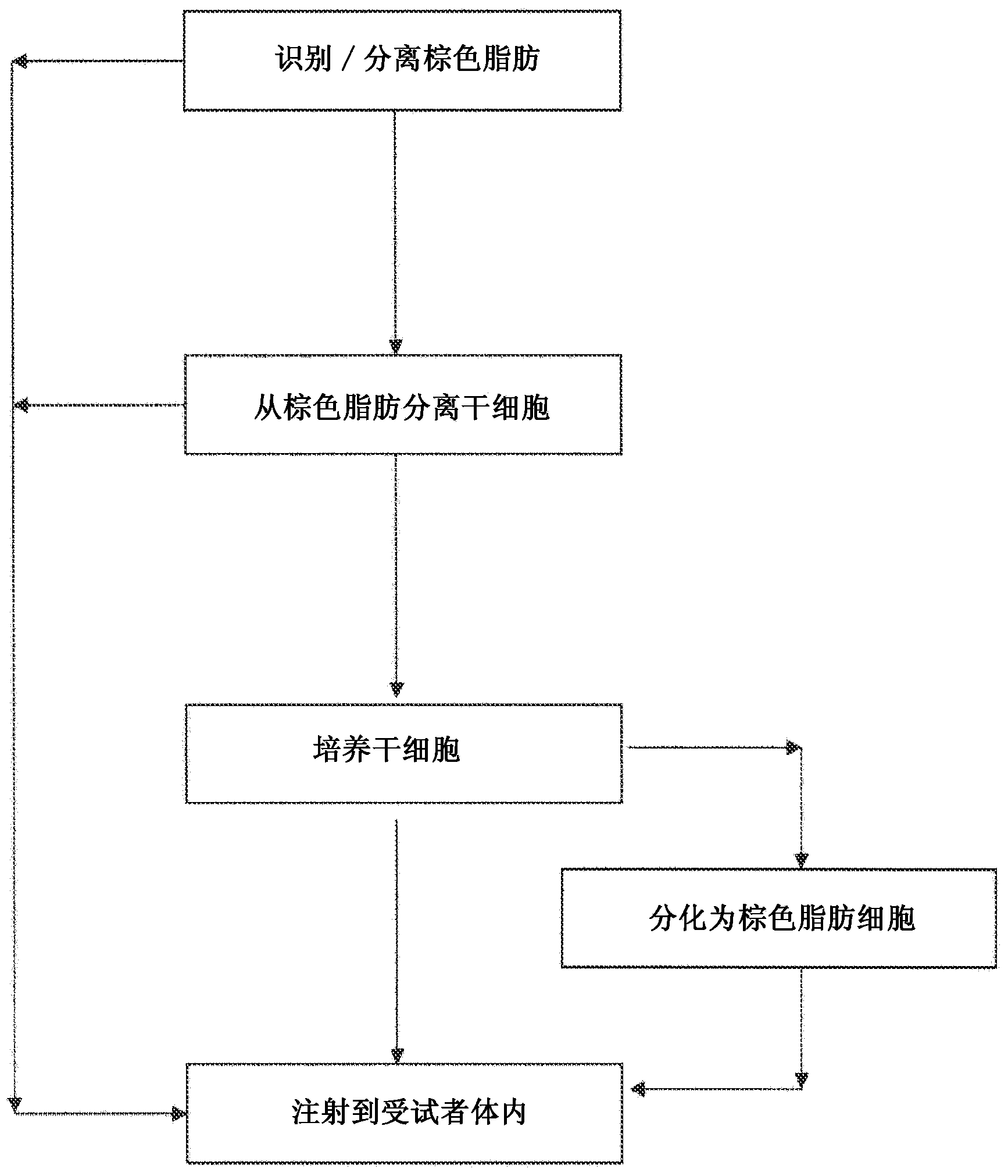
[0117] Embodiment 1-brown fat separation processing
[0118] In one embodiment, the patient first experiences 18 F-fluorodeoxyglucose ( 18 F-FDG) PET-CT scan to identify brown fat deposits. Before undergoing the PET-CT scan, the patient was exposed to a temperature range of 1°C-25°C daily (to increase the 18 Ingestion of F-FDG) for 1-2 hours for a month. Using a needle, biopsies were taken of identified deposits in the supraclavicular and mediastinal regions. Brown fat biopsies were washed three times in DPBS(-) and processed with 0.075%-0.2% collagenase type 1A for 30-60 minutes at 37°C with vigorous shaking or with EDTA for 30 minutes and subjected to 5-30 minutes of incubation. Mechanical tissue destruction. Tissues / cells were washed three times with DPBS(-), and 1x10 6 ~1x10 10 cells were injected into the patient's white fat deposits. Optionally, after enzymatic or mechanical tissue / cell dissociation, the tissue / cells are exposed to cold temperature (0°C-4°C) fo...
Embodiment 2-
[0119] Example 2 - Brown Fat Culture
[0120] In this example, the cells are cultured prior to injection into the patient. The cells obtained from Example 1 were used at 1000 cells / cm 2 The concentration of is inoculated (plate) into the culture medium of the super flask (hyperflask). In this embodiment, the culture medium contains:
[0121] DMEM, low glucose, without phenol red;
[0122] 0.5-20% human platelet lysate;
[0123] 1X NEAA;
[0124] 1X Glutamax;
[0125] 1X Gentamicin;
[0126] 1000 units of heparin.
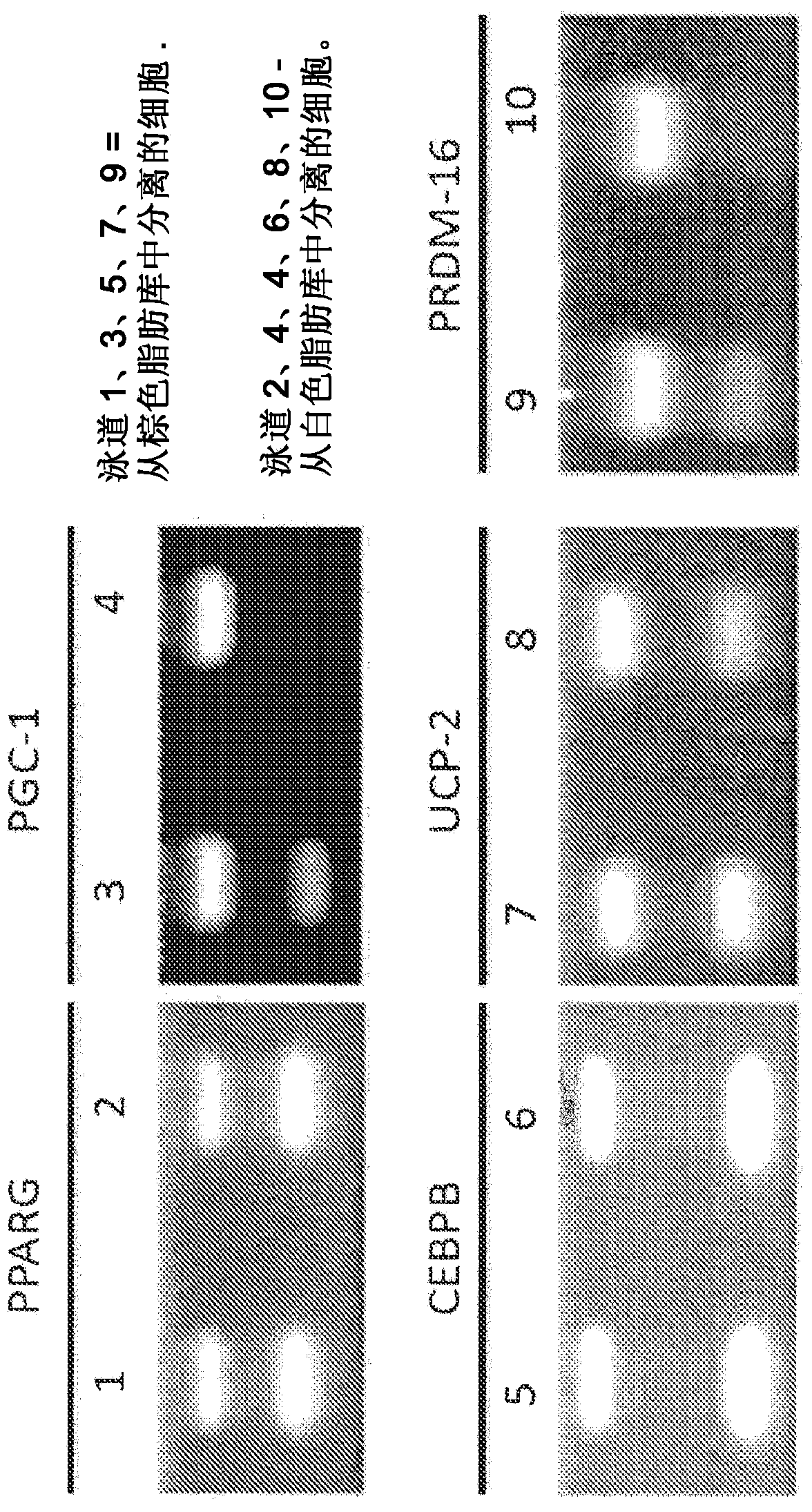
[0127] The cells were cultured for 3-6 weeks. The cells were then characterized using flow cytometry and analyzed for expression of one or more of the following markers: SCA-1, CD34, SSEA1, SSEA4, OCT4, CD31, Wnt5a, telomerase activity, α-SMA, STRO-1, MYF5, PRDM16 or UCP1. The cells were then expanded to 1x10 6 ~1x10 10 concentration to be implanted into the patient's white fat deposits.
Embodiment 3
[0128] Example 3 - Differentiation of cells to brown adipocytes
[0129] In this embodiment, cells obtained from brown adipose deposits of a patient (such as stem cells or precursor brown adipocytes) are differentiated and then injected into the patient. The cells obtained from Example 1 were used at 1000 cells / cm 2 Inoculate into a superflask containing differentiation medium, which in this embodiment comprises:
[0130] DMEM, low glucose, without phenol red;
[0131] 0.5-20% human platelet lysate;
[0132] 1X NEAA;
[0133] 1X Glutamax;
[0134] 1X Gentamicin;
[0135] 1000 units of heparin;
[0136] 10 μg / mL insulin;
[0137] 1 μM dexamethasone;
[0138] 200μM indomethacin;
[0139] 0.5mM isobutylmethylxanthine;
[0140] In an alternative embodiment, the differentiation medium comprises:
[0141] DMEM (low glucose, without phenol red);
[0142] 0.5%-20% human platelet lysate;
[0143] 1X NEAA; 1X Glutamax;
[0144] 1X Gentamicin;
[0145] 1000 units of hepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com