Recombinant baculovirus with surface displaying porcine epidemic diarrhea virus S protein
A technology for porcine epidemic diarrhea and recombinant baculovirus, which is applied in the directions of viruses, viral peptides, antiviral agents, etc., can solve the problems of mutagenicity, low expression level, poor vaccine persistence, etc., and achieves improved display effect, low cost, and preparation. simple craftsmanship
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Construction of recombinant baculovirus
[0042] According to the amino acid sequence of S protein of porcine epidemic diarrhea virus CV777 strain (Genbank Accession Number: AF353511), the nucleic acid sequence of 21-789 amino acids in the S1 region was optimized, and Hangzhou Qingke Zixi Biotechnology Co., Ltd. was entrusted to synthesize the S1 gene containing DNA: SP-S1-TMD, as shown in SEQ ID NO.1. Among them, the 4-60 base sequence is the signal peptide coding sequence (SP) of the baculovirus AcNPV GP64 protein; the 61-2367 base sequence is the PEDV S1 protein coding sequence; the 2368-2577 base sequence is the herpes virus G protein The coding sequence of the transmembrane domain (TMD).
[0043] According to the optimized nucleic acid sequence, DNAstar software was used to design amplification primers P1 (5'-CGCGGATCCATGGTGTCAGCCATCGTG-3') and P2 (5'-CCCAAGCTTTTACTTTCCCAGTCTGTTC-3'), respectively introducing BamHI and HindIII restriction sites. The...
Embodiment 2
[0048] Example 2: Expression and immune identification of S1 protein
[0049] After the recombinant virus sample obtained in Example 1 was titrated by the terminal dilution method, the dose of MOI=1 was used to infect the pre-plated Sf9 cells in the six-well plate. After 72 hours of infection, centrifuge at 1000rpm for 5 minutes, collect the Sf9 cells precipitated from the bottom of the tube, wash the cells twice with PBS, take the cell pellet, prepare protein samples, and perform SDS-PAGE and Western Blotting analysis to verify whether the S1 protein is expressed. Use PEDV S1 antiserum (self-made, 1:100 dilution) as the primary antibody, and the secondary antibody is HRP-goat anti-rabbit IgG antibody (1:10000 dilution), and ECL chemiluminescence is used to detect S1 western blot, and the detection results are as follows image 3 .
[0050] The expected size of the S1 recombinant protein is about 92kD, from image 3 It can be seen that the S1 protein expressed by the Sf9 cel...
Embodiment 3
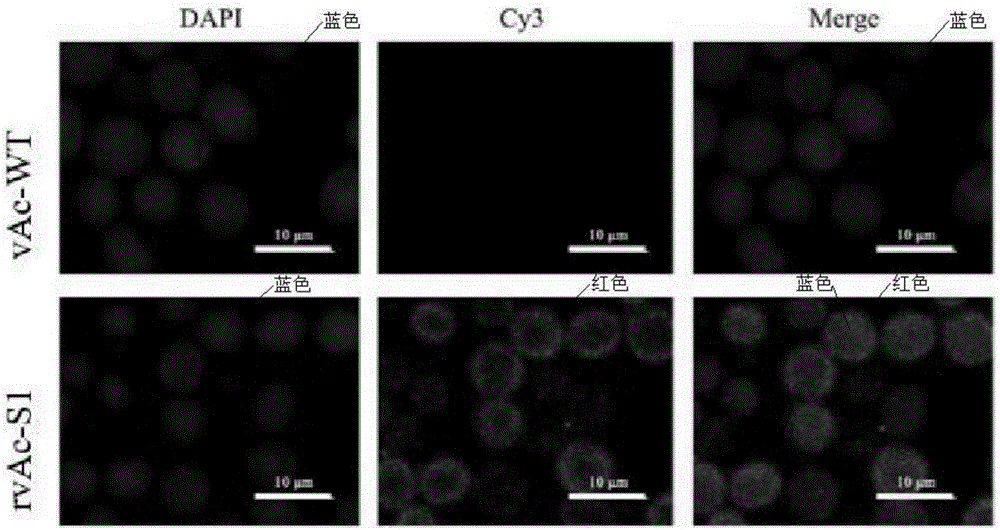
[0051] Example 3: Fluorescent observation and subcellular localization
[0052] In order to further confirm the expression localization of PEDV S1 protein in cells, the rvAc-S1 infected Sf9 cells were observed by indirect immunofluorescence. Sf9 cells were inoculated in a 35mm laser confocal special culture dish (Corning). When the cell confluence reached 50%, the Sf9 cells in the culture dish were inoculated with recombinant baculovirus (MOI=1), and immunofluorescence detection was performed 48 hours after infection. The specific operation is as follows: fix the cells with 4% paraformaldehyde at room temperature for 10 min; permeabilize the cells with 0.2% Triton X-100 for 10 min; overnight at ℃; Cy3-goat anti-rabbit polyclonal antibody (Abcam, 1:500) was incubated at 37°C in the dark for 1 hour; DAPI (Sigma) was used to stain the nucleus for 3 minutes; , pH 7.4) and washed 3 times for 5 min each time, the results were observed and photographed under a confocal laser scannin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com