Cryptosporidium parvum divalent protein vaccine and preparation method thereof
A Cryptosporidium parvum and protein vaccine technology, which is applied in the field of infectious disease vaccine preparation, can solve the problem of no cryptosporidium killing effect, and achieve good prevention of Cryptosporidium parvum in animals, enhanced immune protection effect, and strong immune response The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
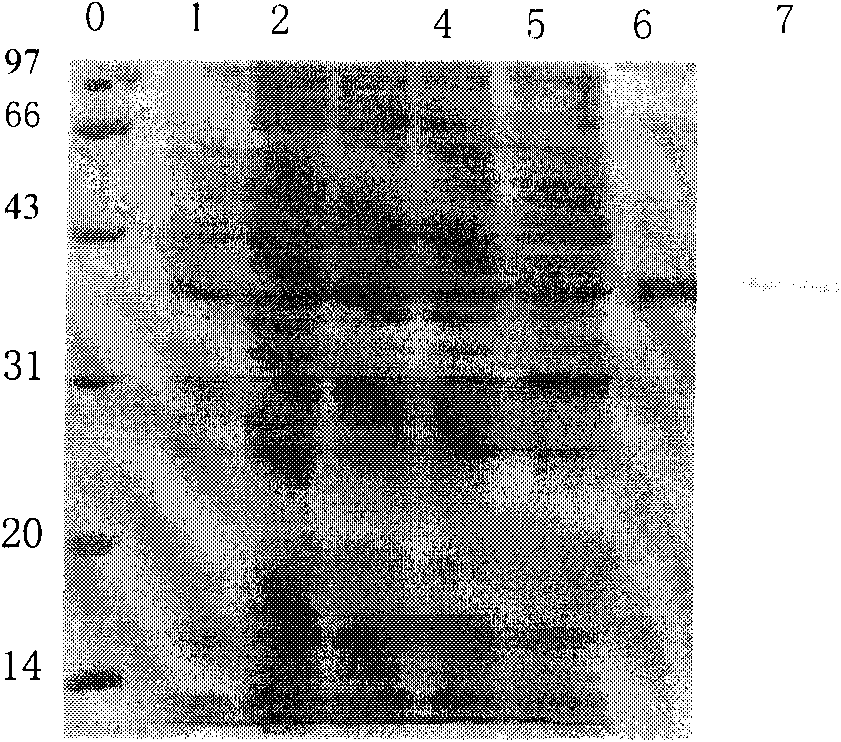
Image
Examples
Embodiment 1
[0017] Preparation of bivalent protein vaccine against Cryptosporidium parvum
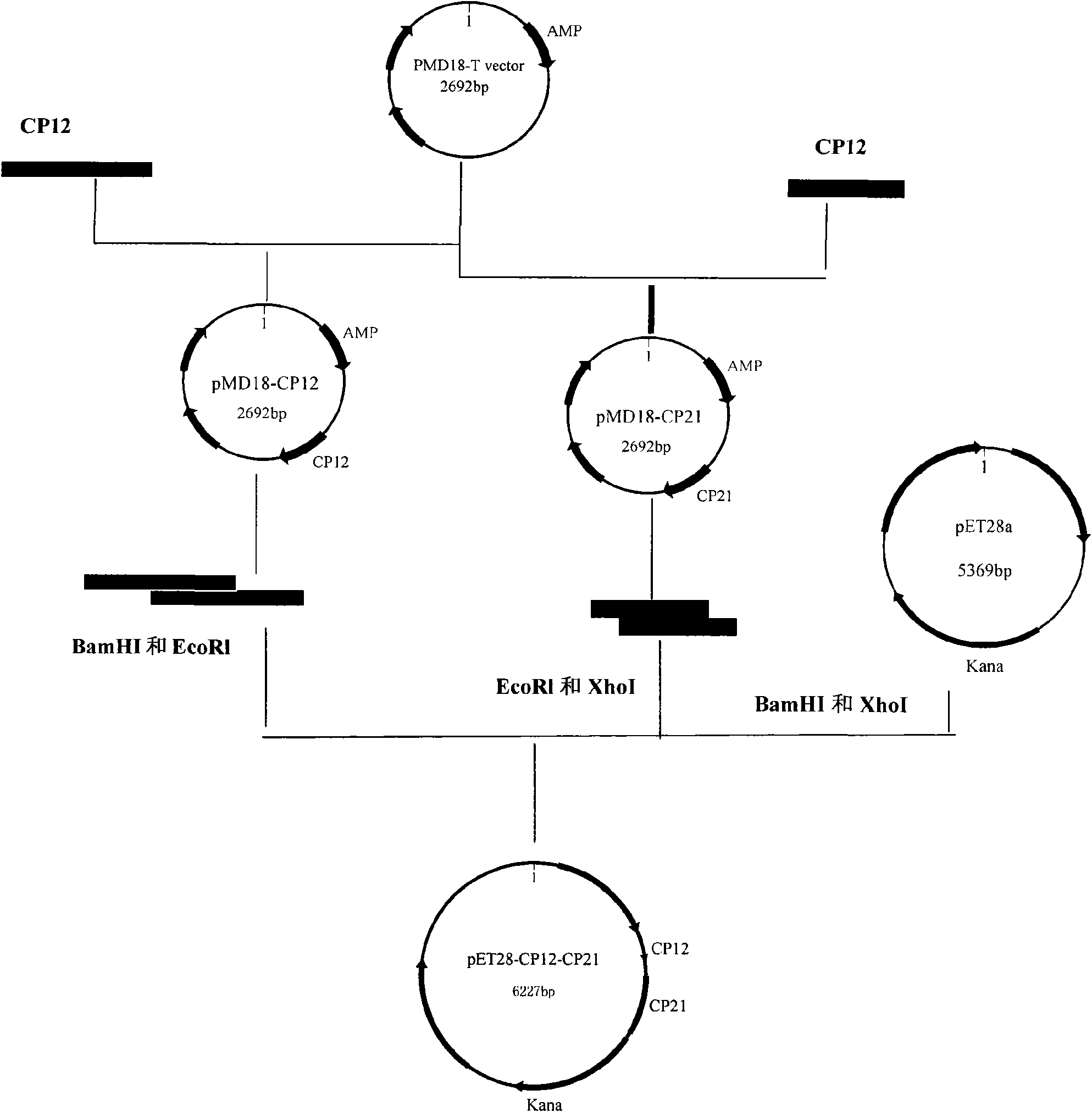
[0018] The present invention takes the immune-related protein genes CP12 and CP21 of Cryptosporidium parvum as an example to construct the recombinant prokaryotic expression vector.
[0019] The preparation steps of Cryptosporidium parvum bivalent protein vaccine:
[0020] Refer to the open reading frames of CP12 and CP21 gene sequences and the physical map of prokaryotic expression vector pET28a to design primers and introduce restriction sites.
[0021] CP12 upstream primer:
[0022] QF1: 5'-CT GGATCC ATGTCAGATGCATCAATA-3'; where the 5' end contains a BamHI site;
[0023] Downstream primer QR1: 5'-GGGGC GAATTC TATTTGTTCATTCATCTG-3'; 5' end contains an EcoRI site.
[0024] CP21 upstream primer:
[0025] QF2: 5'-CC GAATTC GGTGGCGGTGGCTCGATGTCTAAAAAGAGCAT-3', the 5' end contains EcoRI site and Linker sequence;
[0026] Downstream primer QR2: 5'-CTGGCT CTCGAG CTATTCATCTGTTTGAAC-3'; 5' en...
Embodiment 2
[0029] Uses of Cryptosporidium parvum Bivalent Protein Vaccine
[0030] 1. Application of Cryptosporidium parvum bivalent protein vaccine
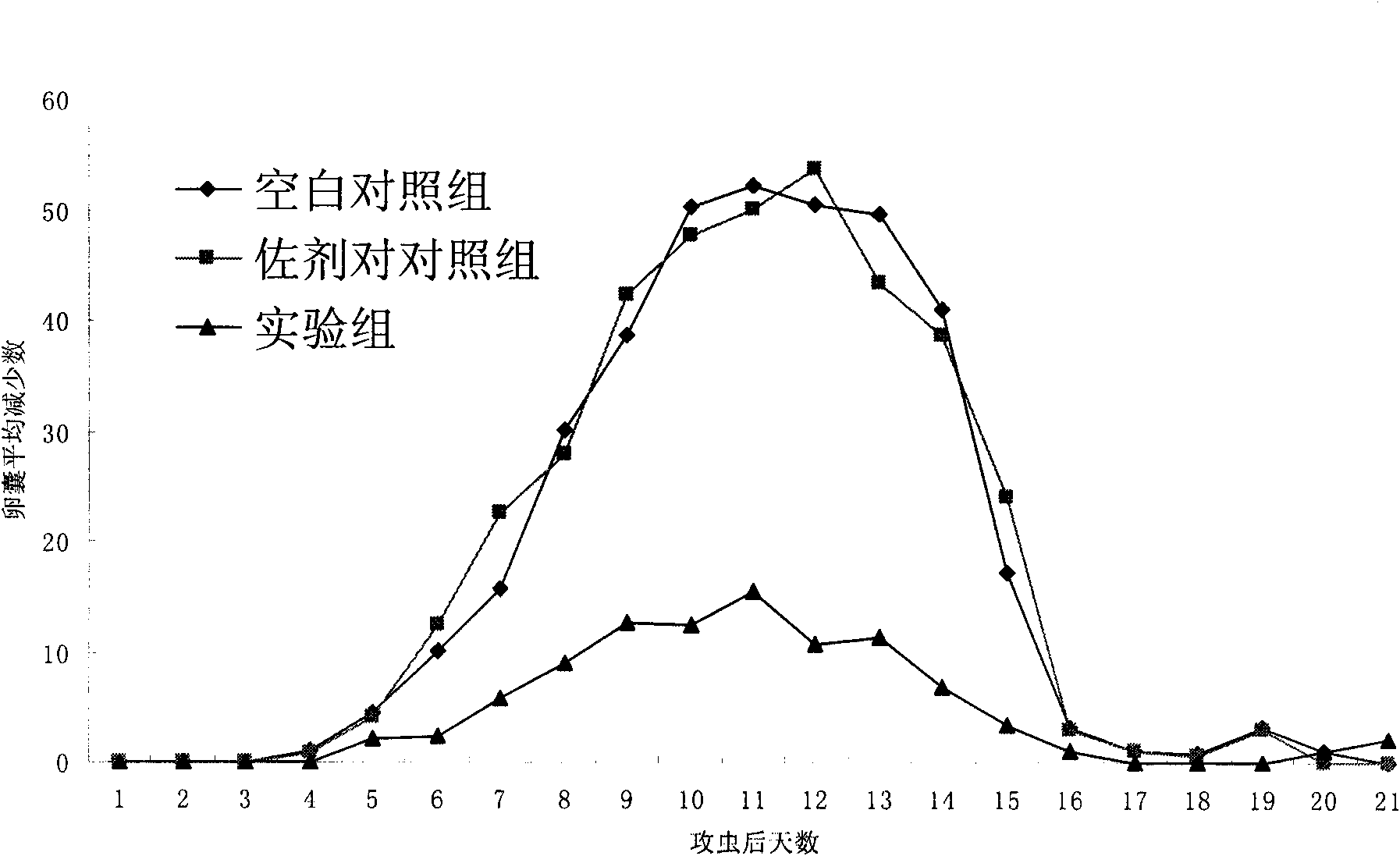
[0031] Divide 60 4-6 weeks old, 18-22g female clean BALB / c mice into 3 groups, 20 mice in each group. The experimental group was intraperitoneally injected with recombinant protein, and immunized three times with an interval of 2 weeks between each time. For the first immunization, 0.1 mL antigen solution (containing 20 μg recombinant protein) was emulsified with an equal volume of Freund’s complete adjuvant and injected intraperitoneally; for the second and third immunizations, 0.1 mL antigen solution (containing 20 μg recombinant protein) was mixed with an equal volume of The incomplete adjuvant was emulsified and injected intraperitoneally. The adjuvant control group (Negative control B) was intraperitoneally injected with the same amount of Freund's adjuvant as the test group, the first injection of Freund's complete adjuvant, the sec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com