Patents
Literature
59 results about "Cryptosporidium parvum" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cryptosporidium parvum is one of several species that cause cryptosporidiosis, a parasitic disease of the mammalian intestinal tract. Primary symptoms of C. parvum infection are acute, watery, and nonbloody diarrhea. C. parvum infection is of particular concern in immunocompromised patients, where diarrhea can reach 10–15 l per day. Other symptoms may include anorexia, nausea/vomiting, and abdominal pain. Extra-intestinal sites include the lung, liver, and gall bladder, where it causes respiratory cryptosporidosis, hepatitis, and cholecystitis, respectively.
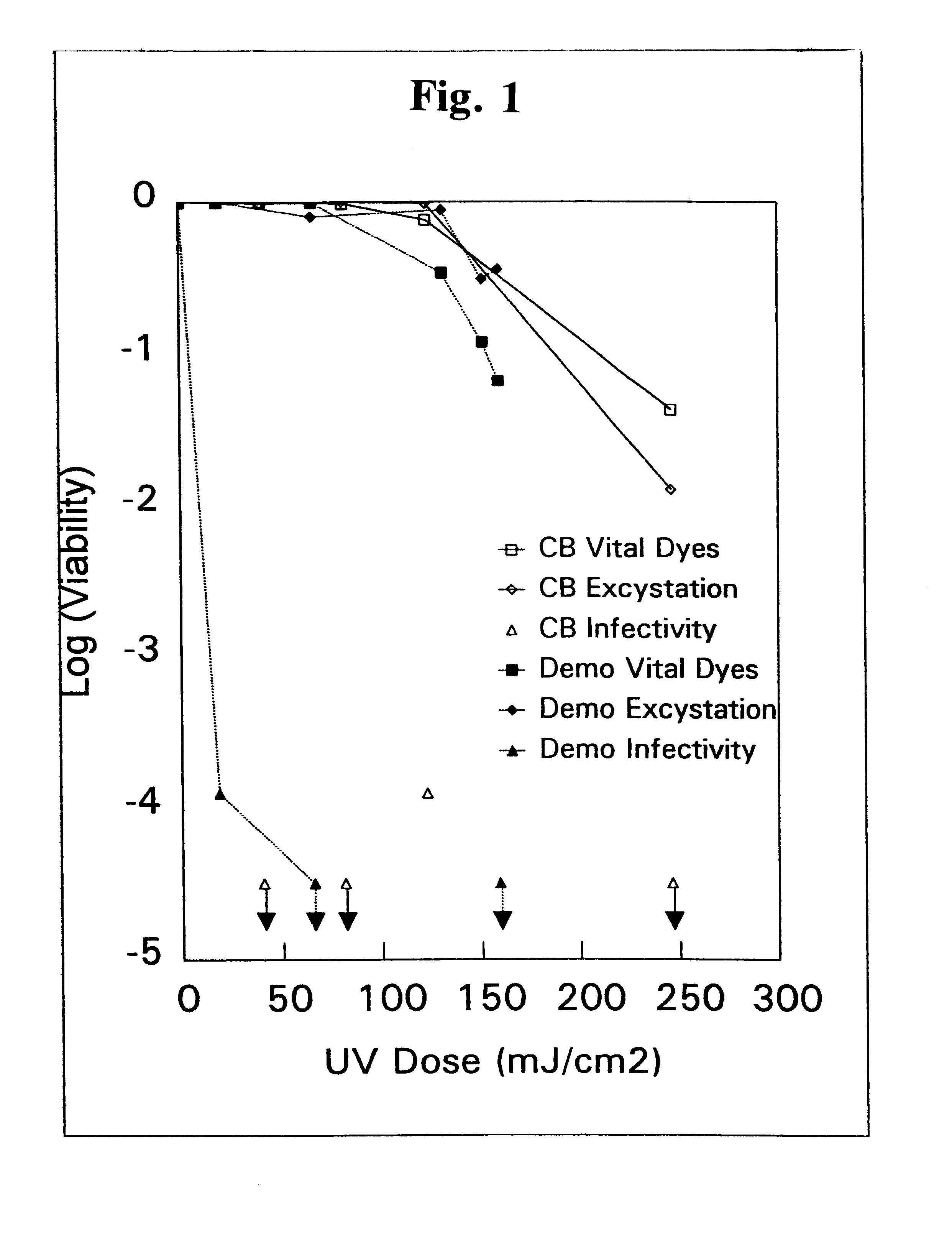
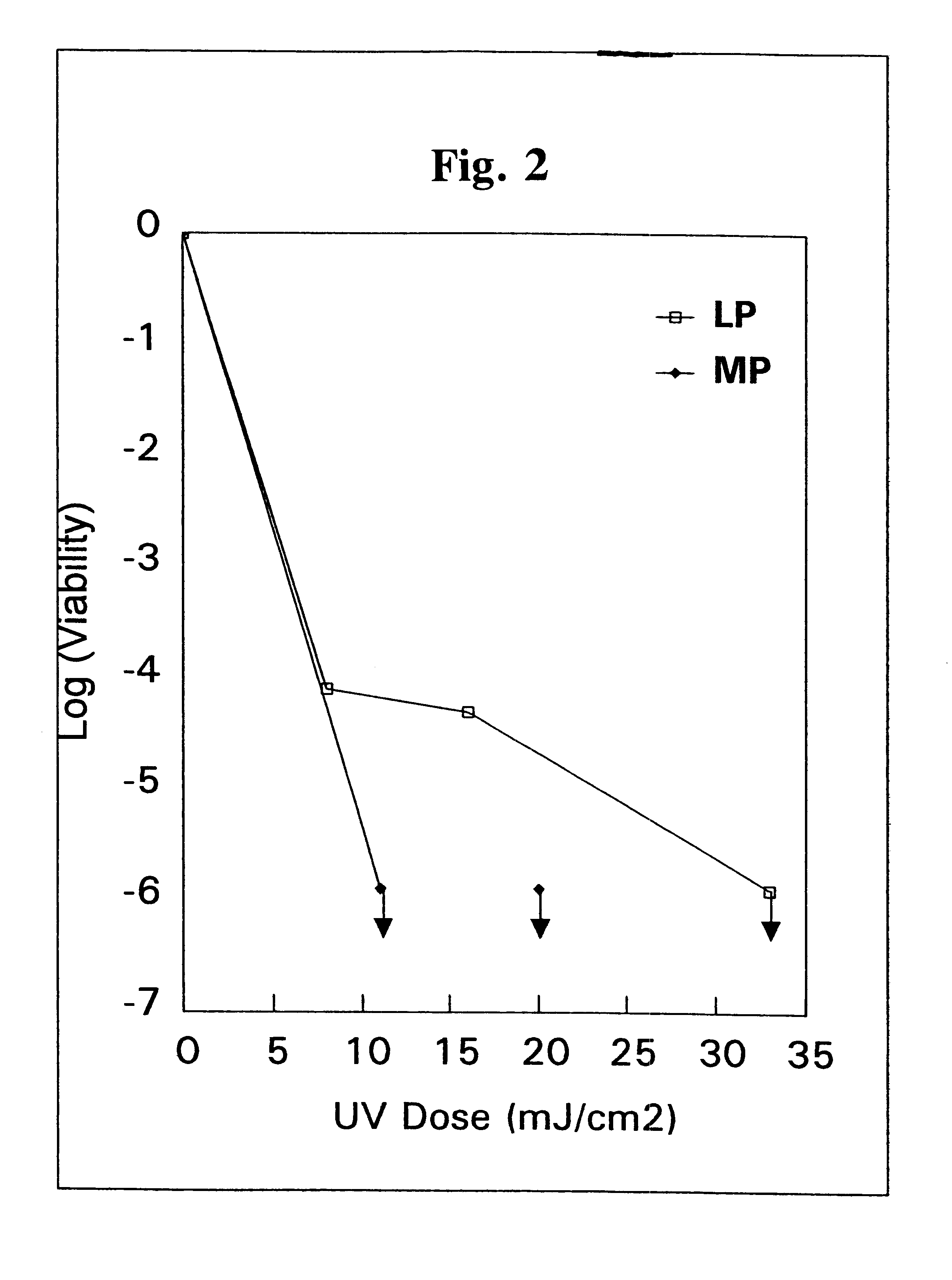
Method for the inactivation of cryptosporidium parvum using ultraviolet light
InactiveUS6565803B1Water/sewage treatment by irradiationSpecific water treatment objectivesCryptosporidium parvumUltraviolet lights
A method for the inactivation of Cryptosporidium oocysts, Giardia cysts and similar organisms comprising irradiating water with ultraviolet light in doses of from about 1 mJ / cm2 to about 175 mJ / cm2.
Owner:CALGON CARBON
Tetracycline compounds for treatment of cryptosporidium parvum related disorders
Methods and compositions for treating Cryptosporidium parvum related disorders in a mammal are discussed. Several novel tetracycline compounds useful for treating Cryptosporidium parvum related disorders are also included.
Owner:TRUSTEES OF TUFTS COLLEGE
Tetracycline compounds for treatment of cryptosporidium parvum related disorders
Methods and compositions for treating Cryptosporidium parvum related disorders in a mammal are discussed. Several novel tetracycline compounds useful for treating Cryptosporidium parvum related disorders are also included.
Owner:TRUSTEES OF TUFTS COLLEGE
Lactobacillus reuteri to inhibit cryptosporidiosis in mammals
InactiveUS6103227AAvoid infectionImprove the immunityBiocideBacteriaChronic diarrheaImmune compromised
Cryptosporidium parvum (the cause of cryptosporidiosis) has become one of the most common enteropathogens causing diarrhea worldwide. Symptoms associated with cryptosporidiosis are very debilitating especially in the immunocompromised subject (e.g., AIDS patient). Clinical features include severe, chronic diarrhea, abdominal cramps, fatigue, weight loss, etc. which lead to increased health care costs and increased mortality. There is disclosed herein a method of inhibiting the severity of Cryptosporidium parvum infection by enterally administering a therapeutically effective amount of Lactobacillus reuteri.
Owner:WOLF BRYAN WARREN +1
Multiplex rt-pcr/pcr for simultaneous detection of bovine coronavirus, bovine rotavirus, cryptosporidium parvum, and escherichia coli
InactiveUS20050026144A1Rapid and sensitive and specificRapid and sensitive and detectionSugar derivativesMicrobiological testing/measurementBovine rotavirusEscherichia coli
The present invention provides a multiplex RT-PCR / PCR method, which enables in a single assay the simultaneous detection of any combination of bovine rotavirus, bovine coronavirus, Cryptosporidium parvum, and optionally, Escherichia coli strains producing K99 pili or heat-stable enterotoxin STa.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Compositions And Methods For Treating Toxoplasmosis, Cryptosporidiosis, And Other Apicomplexan Protozoan Related Diseases
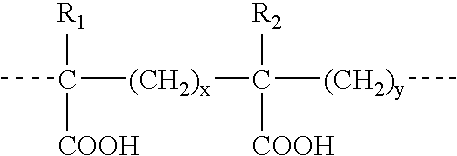
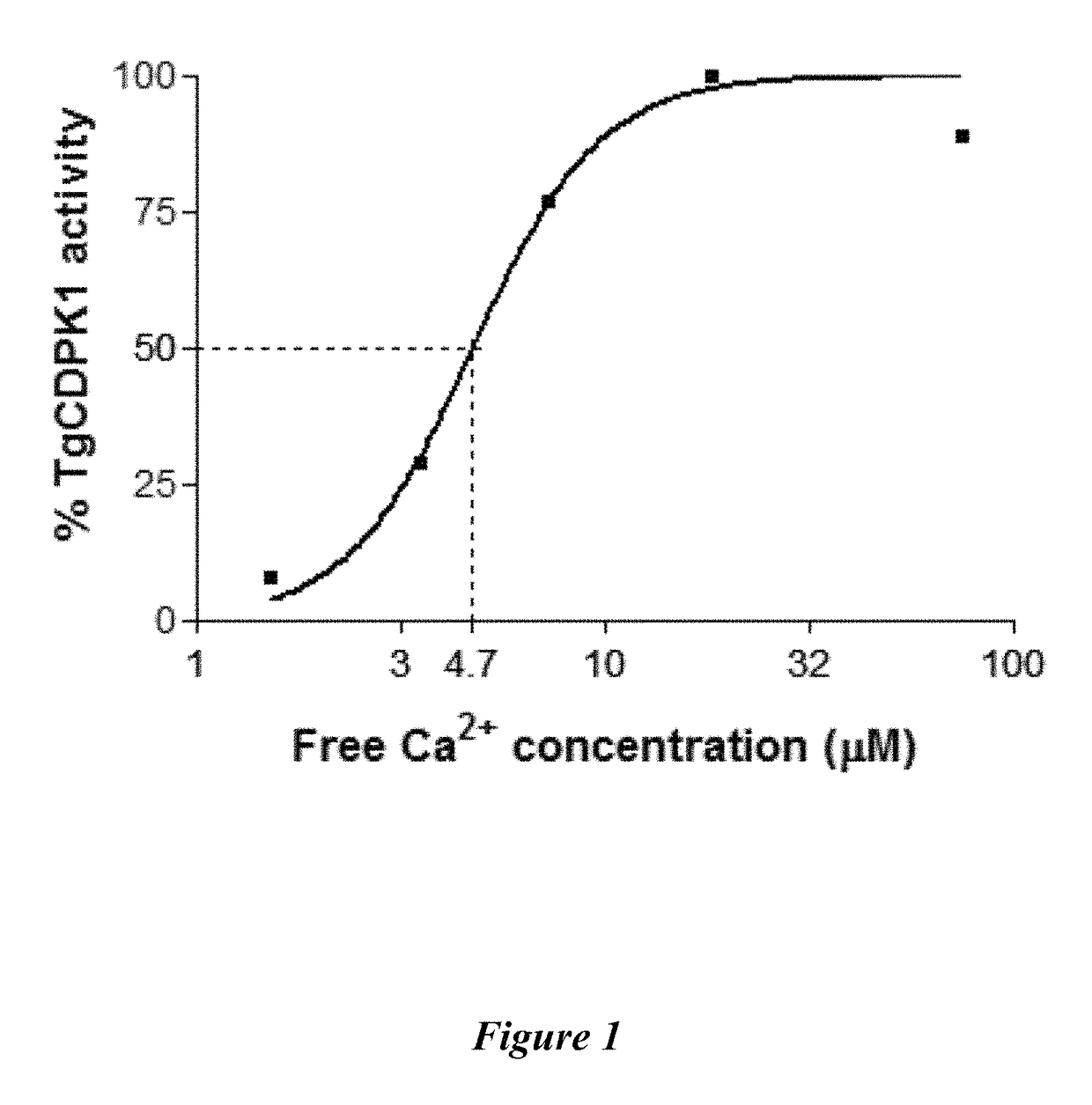
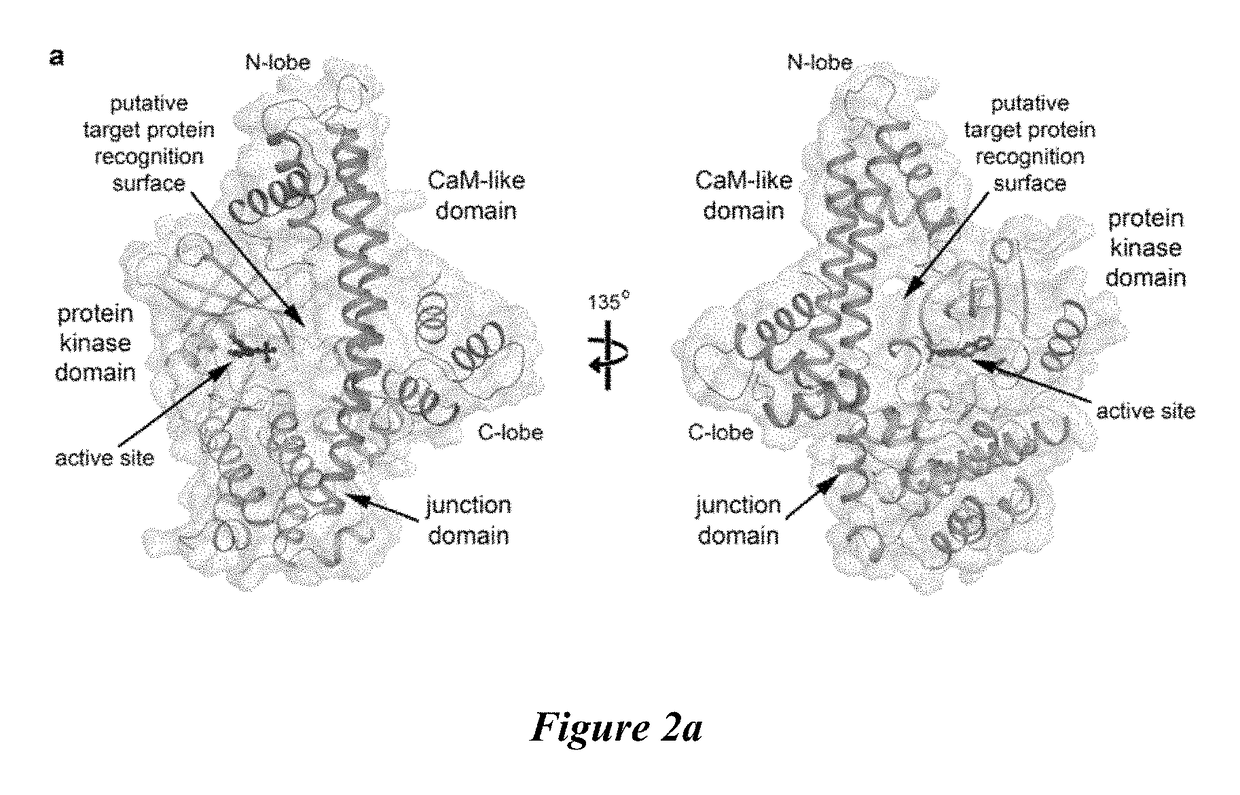
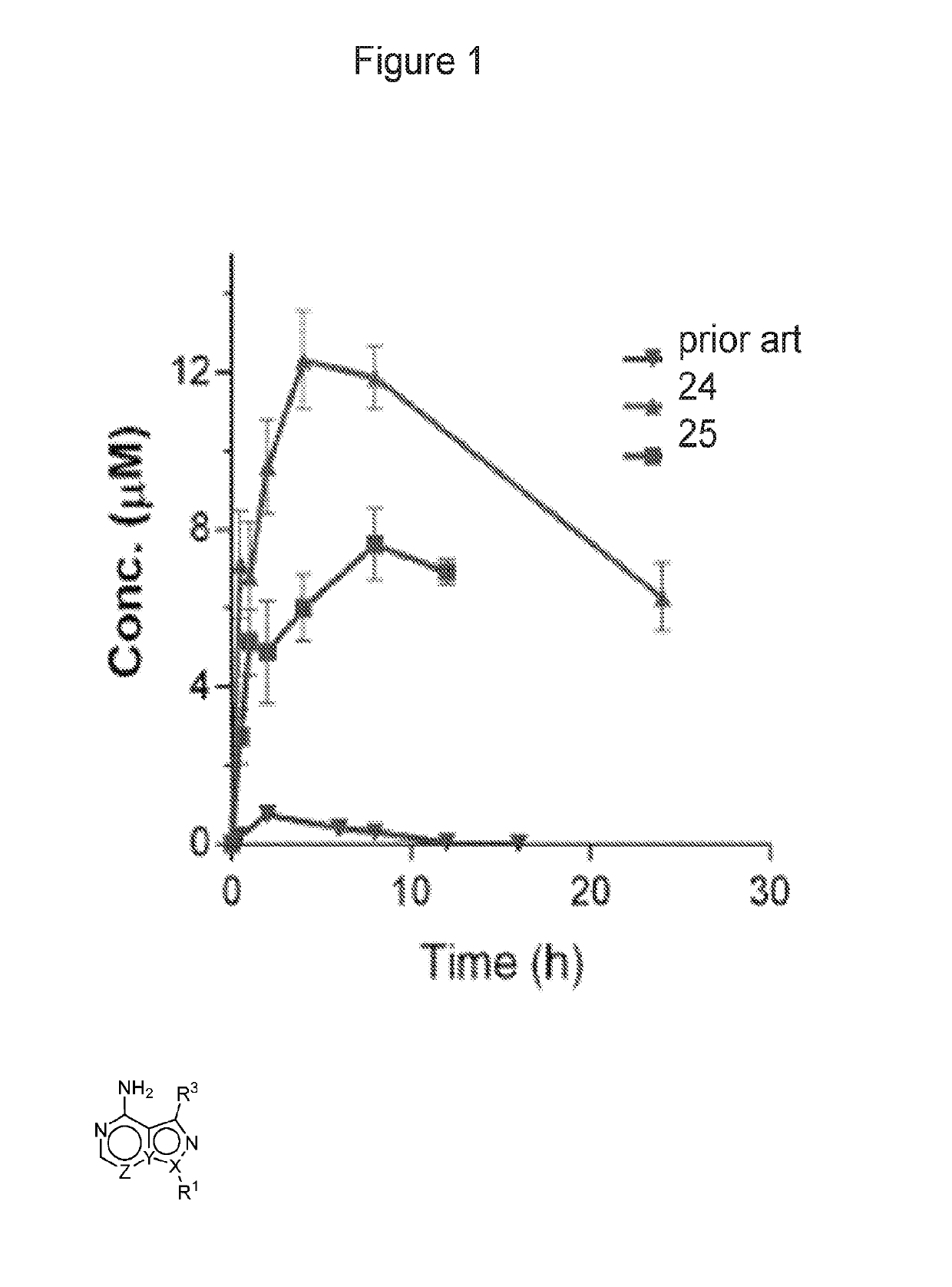
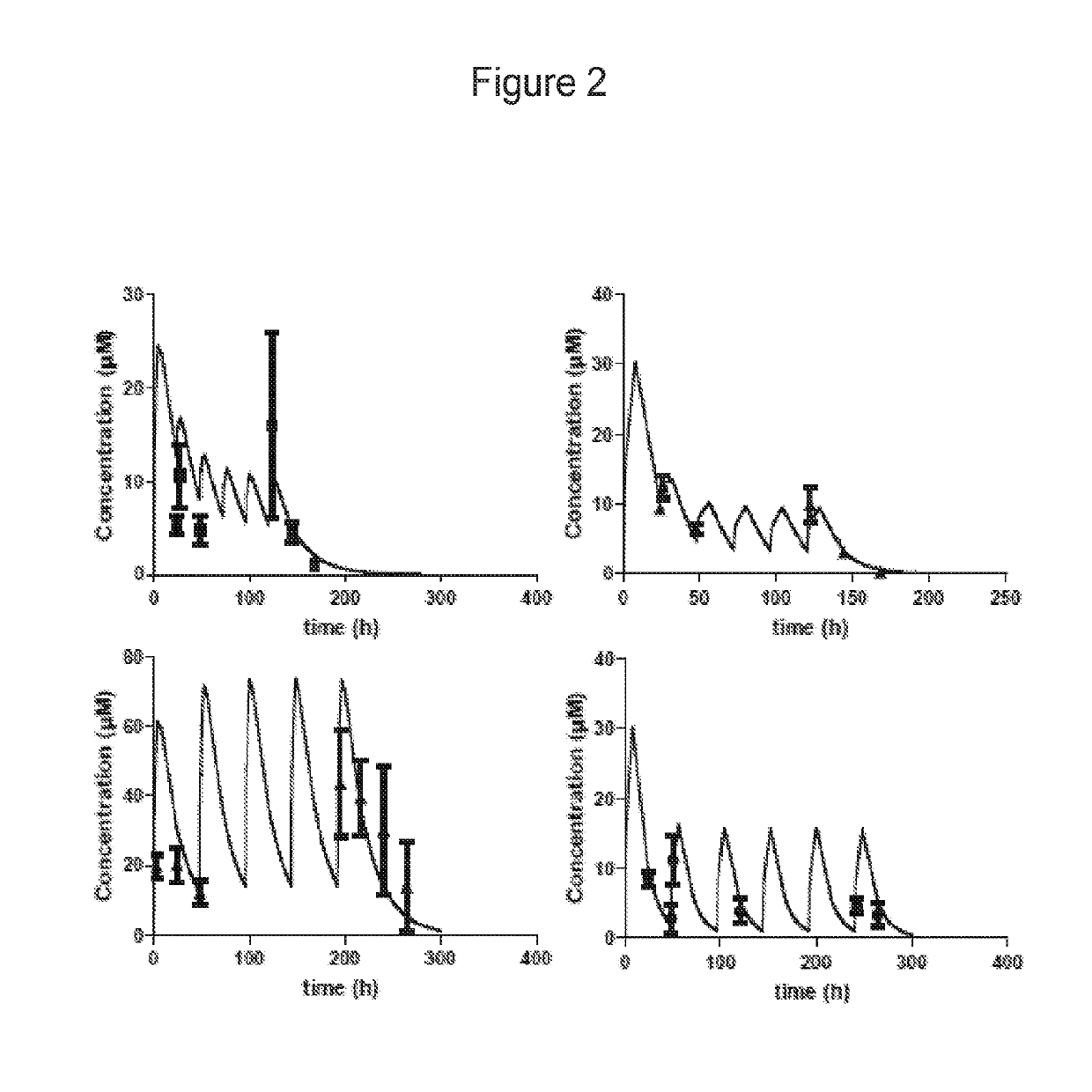
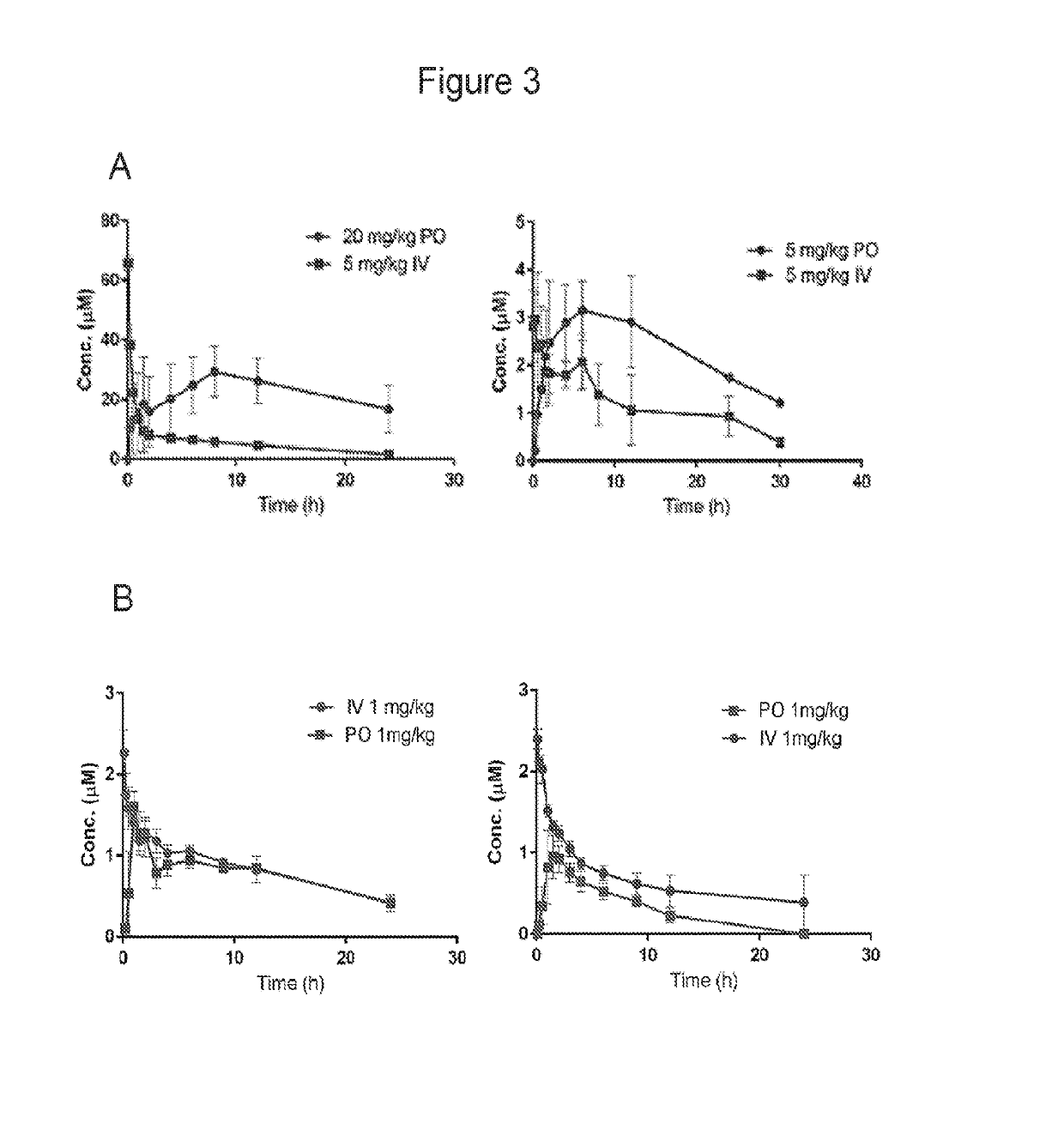
Compositions and methods for the treatment of toxoplasmosis, caused by the infectious eukaryotic parasite Toxoplasma gondii (T. gondii) and for the treatment of cryptosporidiosis, caused by the infectious eukaryotic parasites Cryptosporidium parvum (C. parvum) and Cryptosporidium hominus (C. hominus) are described. In particular, the present disclosure is directed to compositions and methods for inhibiting either T. gondii calcium dependent protein kinases (TgCDPKs) or C. parvum and C. hominus calcium dependent protein kinases (CpCDPKs) using pyrazolopyrimidine and / or imidazo[1,5-a]pyrazine inhibitors, of the formula,wherein the variables X, Y, Z, L, R1, and R3 are defined herein.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
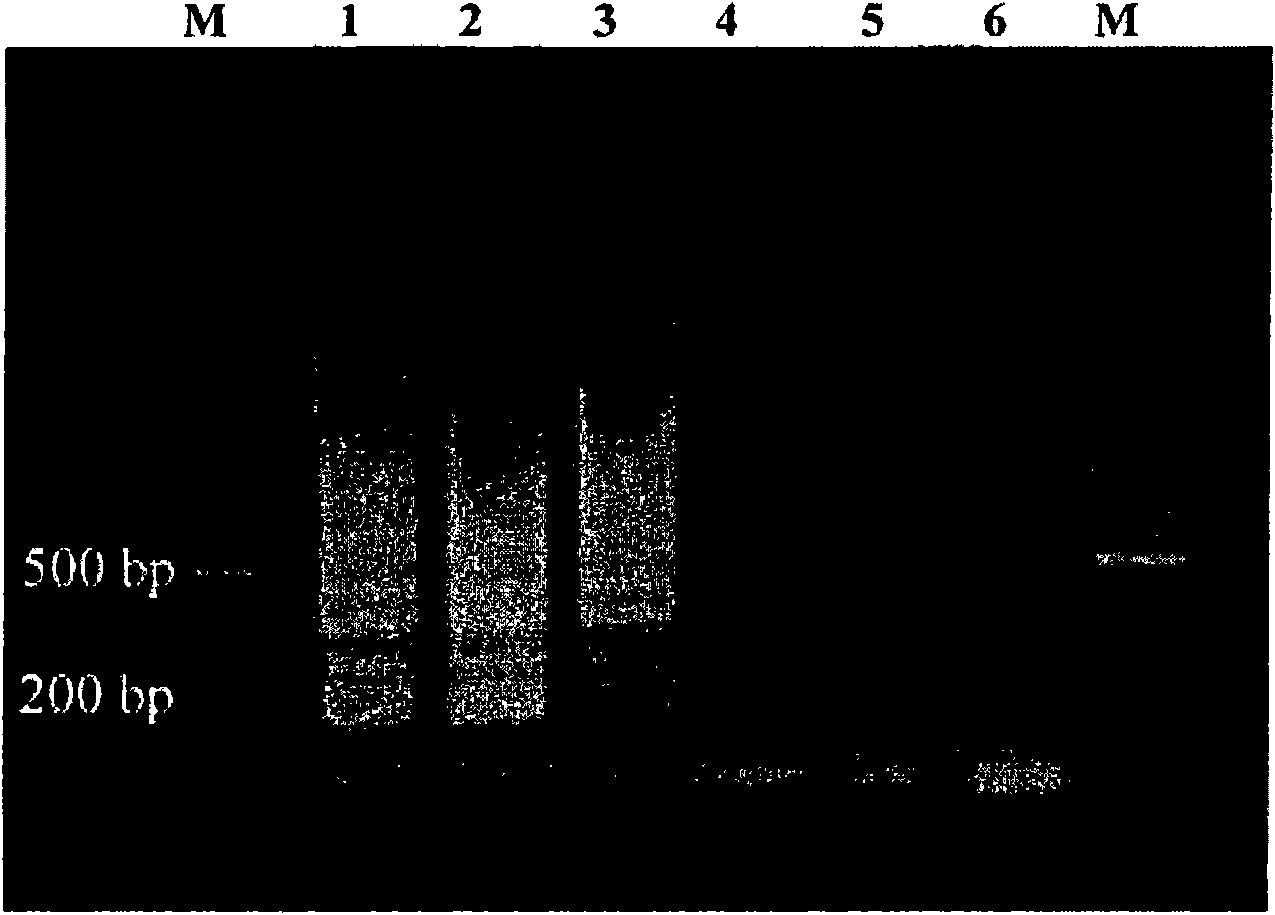
Cryptosporidium and cryptosporidium parvum specific PCR detecting reagent kit and detecting method
InactiveCN101353690ARigorous designEasy to operateMicrobiological testing/measurementAgainst vector-borne diseasesPositive controlCryptosporidium infection
The invention provides a peculiar PCR detection kit of Cryptosporidium and micro Cryptosporidium, comprising a DNA lysis solution, a PCR reaction solution, a peculiar primer of the Cryptosporidium and the micro Cryptosporidium and positive control genomic DNA of the micro Cryptosporidium. According to the diagnosing sequence for analyzing Cryptosporidium and species peculiarities, a Cryptosporidium and species peculiar primer are designed by utilizing a peculiar detection method of PCR, the sensibility of the primer is improved by optimizing and amplifying conditions, the amplifying result can be directly observed by electrophoretic analysis. The kit of the invention has strict design, simple and easy operation, high sensibility and strong peculiarity, can simultaneously identify the Cryptosporidium infection of Cryptosporidium and species peculiarities so as to obtain an accurate and objective judgment result.
Owner:JILIN UNIV
Compositions, methods and kits for determining the presence of Cryptosporidium parvum organisms in a test sample
The present invention describes novel oligonucleotides targeted to nucleic acid sequences derived from Cryptosporidium organisms, and Cryptosporidium parvum organisms in particular, which are useful for determining the presence of Cryptosporidium organisms in a test sample. The oligonucleotides of the present invention include hybridization assay probes, helper probes and amplification primers. The present invention further describes a novel method for obtaining purified ribonucleic acid from viable oocysts.
Owner:GEN PROBE INC
Various important aquagenic zoonoses protozoa simultaneous assay kit and preparation method thereof
InactiveCN102154496AAccurate detectionIncreased sensitivityMicrobiological testing/measurementAgainst vector-borne diseasesProtozoaGondii toxoplasma
The invention discloses a various important aquagenic zoonoses protozoa simultaneous assay kit, and further provides a preparation method of the kit. A primer and a probe are designed according to the special gene sequence of the giardia lamblia, the cryptosporidium parvum and the toxoplasma gondii by a multi-fluorescence quantitative PCR (polymerase chain reaction) specific assay method, the sensitivity is improved by optimizing the fluorescence quantitative PCR reaction system and condition, the amplification can be performed, and an amplification result can be directly and timely observed.The invention can be used for fast and exactly assaying three important aquagenic zoonoses protozoa, and has the characteristics of being precise in design, simple and easy to operate, high in sensitivity, high in specificity, exact and objective in judgment, and the like.
Owner:JILIN UNIV
Compositions and vaccines containing antigen(s) of Cryptosporidium parvum and of another pathogen
InactiveUS20050106163A1Improve vaccinationImproved and useful productionBacterial antigen ingredientsProtozoa antigen ingredientsEpitopeMammal
Combination compositions including C. parvum antigen(s) or epitope(s) of interest with at least one other antigen or epitope of interest from a pathogen that causes enteric infection and / or symptoms and / or recombinant(s) and / or vector(s) and / or plasmid(s) expressing such antigen(s) or epitope(s) of interest and administration of such compositions such as to pregnant mammals and / or newborn or young mammals, for instance, pregnant cows and / or calves such as within the first month of birth, are disclosed and claimed.
Owner:MERIAL LTD
Cryptosporidium parvum divalent protein vaccine and preparation method thereof
InactiveCN101658667AGood immune protectionStrong immune responseAntiparasitic agentsAntibody medical ingredientsProtective antigenDisease
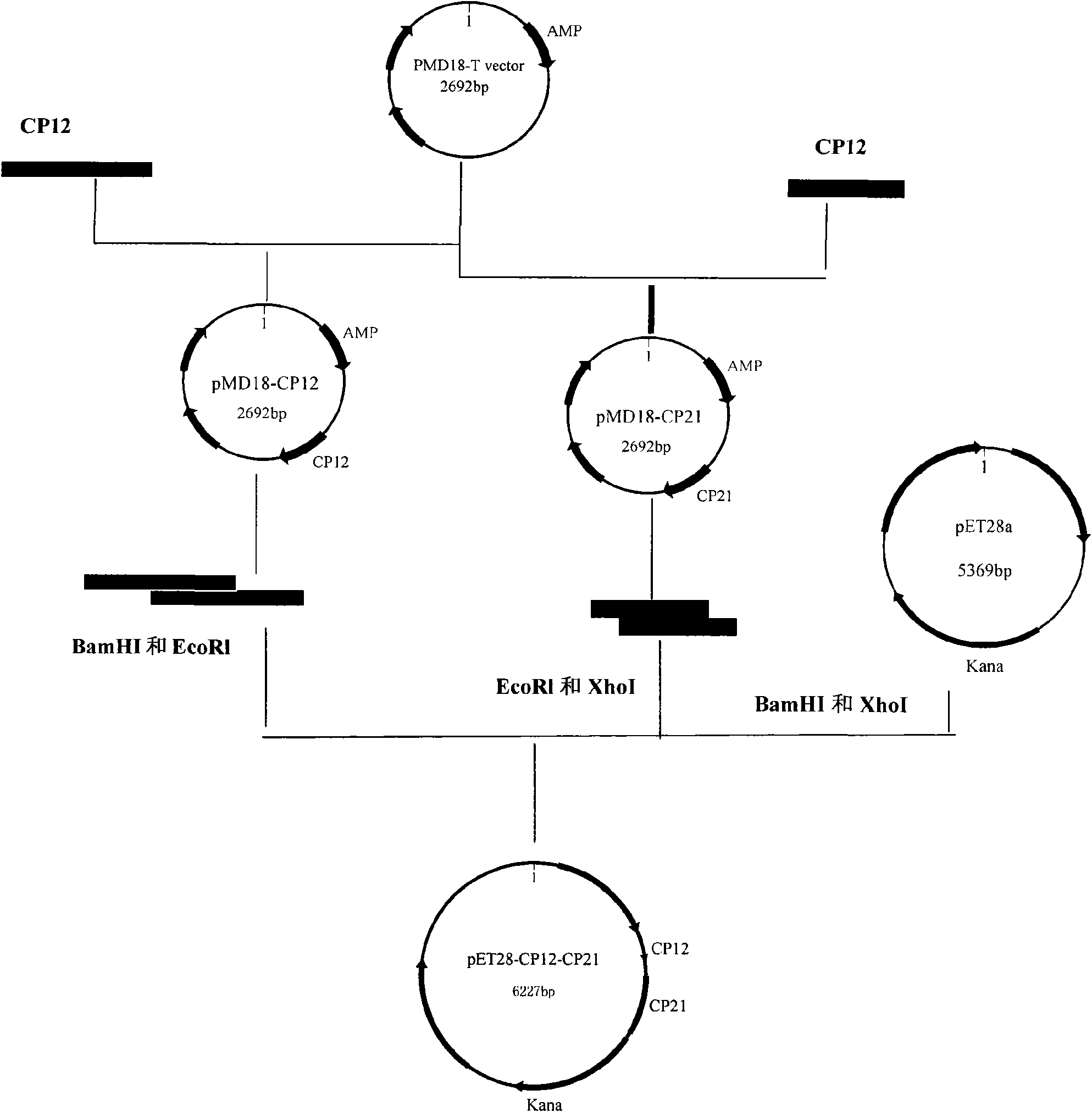
The invention provides a cryptosporidium parvum divalent protein vaccine, which is characterized in that prokaryotic expression vector pET28a is taken as a carrier, and two cryptosporidium parvum protective antigen genes are inserted in series to the multiple cloning sites of the prokaryotic expression pET28a, thus obtaining the cryptosporidium parvum divalent protein vaccine. The vaccine selectstwo advantageous protective antigens, combines the advantages of the two antigens, and can induce stronger functions of cellular immunity and humoral immunity, thus achieving the purposes of preventing cryptosporidium parvum disease of animal better.
Owner:JILIN UNIV
Cryptosporidium parvum immune colloidal gold detection test paper strip and production method thereof
InactiveCN102183653AImprove the effect of prevention and controlSuitable for testingImmunoglobulinsFermentationDiseaseProtein.monoclonal
The invention provides a cryptosporidium parvum immune colloidal gold detection test paper strip and a production method thereof. The test paper strip comprises a nitrocellulose membrane, a gold marking pad and an absorption pad, wherein the gold marking pad and the absorption pad are arranged at two ends of the nitrocellulose membrane; the upper end of the gold marking pad is a sample pad, and the gold marking pad is coated with a purified CSpV-S protein monoclonal antibody colloidal gold coupling marker, a detection line is coated with a purified monoclonal antibody, a quality control line is coated with a goat anti mouse IgG antibody, and the absorption pad is attached to the side of the quality control line. The research combines the enzyme-immunoassay principle and colloidal gold chromatography to prepare the colloidal gold test paper strip for detecting C.parvum, which is going to be applied to the clinic to improve the prevention and treatment ability of C.parvum, and the invention has the advantages of simple and rapid operation, clear detection results and easy judgment, high specificity, high sensitivity, no need of instruments and apparatuses or just need of simple instruments and the like, and therefore is especially suitable for clinic sample detection use in sites where diseases occur, outpatient departments, places having no experimental conditions and the like.
Owner:JILIN UNIV
Method for detecting cryptosporidium parvum and detection kit
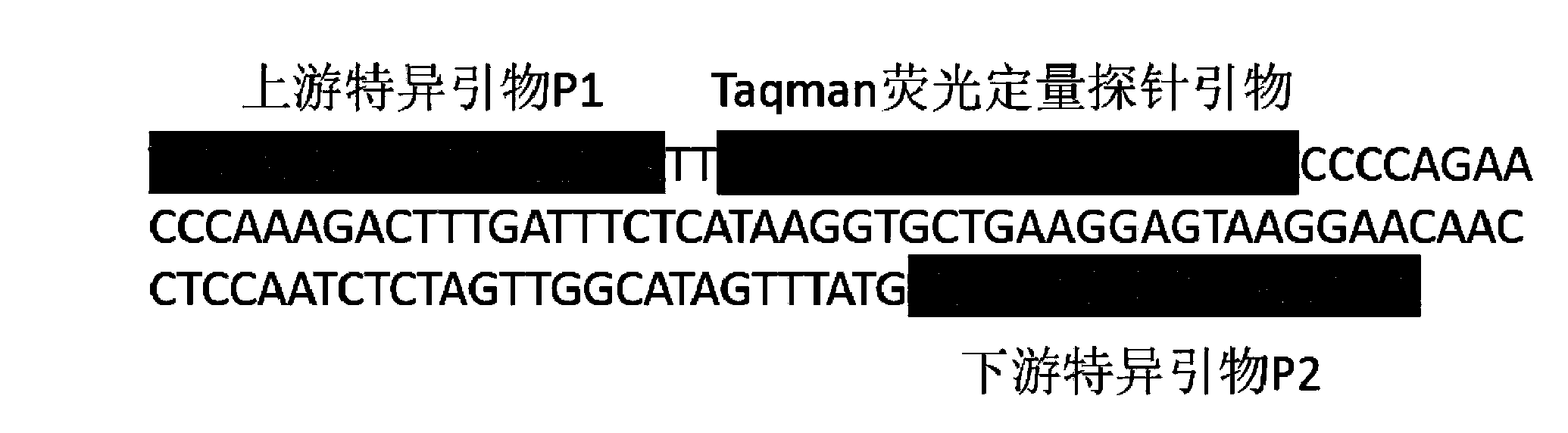
ActiveCN103898203AEfficient separationAccurate and rapid separation and purification methodMicrobiological testing/measurementMicroorganism based processesForward primerMagnetic bead
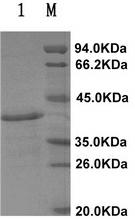
The invention provides a method for detecting cryptosporidium parvum and a detection kit. The detection method comprises the following steps: (1) separating and purifying a sample to be detected by immunomagnetic beads to prepare suspension containing cryptosporidium parvum; and (2) detecting the suspension containing cryptosporidium parvum prepared in the step (1) by fluorogenic quantitative PCR (polymerase chain reaction). The detection kit comprises a reaction system containing biotinylated cryptosporidium parvum Cp23 monoclonal antibody coated streptomycin magnetic beads in the detection method, a specific forward primer of cryptosporidium parvum, a specific reverse primer and a Taqman fluorogenic quantitative probe primer. According to the cryptosporidium parvum detection method, the sensitivity is 100 times higher than that of a traditional method, and is far beyond that of the traditional method.
Owner:INST OF ZOOLOGY CHINESE ACAD OF SCI
Compositions and methods for treating toxoplasmosis, cryptosporidiosis, and other apicomplexan protozoan related diseases
Compositions and methods for the treatment of toxoplasmosis, caused by the infectious eukaryotic parasite Toxoplasma gondii (T. gondii) and for the treatment of cryptosporidiosis, caused by the infectious eukaryotic parasites Cryptosporidium parvum (C. parvum) and Cryptosporidium hominus (C. hominus) are described. In particular, the present disclosure is directed to compositions and methods for inhibiting either T. gondii calcium dependent protein kinases (TgCDPKs) or C. parvum and C. hominus calcium dependent protein kinases (CpCDPKs) using pyrazolopyrimidine and / or imidazo[1,5-a]pyrazine inhibitors, of the formula,wherein the variables X, Y, Z, L, R1, and R3 are defined herein.
Owner:UNIV OF WASHINGTON CENT FOR COMMERICIALIZATION
A method of detecting cryptosporidium
InactiveCN101970690AMicrobiological testing/measurementAgainst vector-borne diseasesBiotechnologyBiological body
A method for the detection and / or identification of Cryptosporidium organisms in general, and one or more of C. hominis,C. parvum and C. meleagridis organisms in particular and / or nucleic acid sequences and to hybridization assay probes, helper probes, amplification primers, nucleic acid compositions, probe mixes, methods and kits useful for determining the presence of Cryptosporidium organisms in general, and one or more of C. hominis, C. parvum and C. meleagridis organisms in particular, in a test sample of water, faeces, food or other sample media.
Owner:SYDNEY WATER CORP
Cryptosporidium parvum recombinant antigen for diagnosis
InactiveCN103740733AImprove featuresGenetic engineeringFermentationProtein detectionCryptosporidium parvum
The invention discloses a cryptosporidium parvum recombinant antigen gene (i) MUCIN ( / i) and an expressed fusion protein recombinant antigen rMUCIN. 520 parts of healthy human serum are randomly taken, the recombinant antigens rMUCIN and rCp23 are used as detection antigens, a human serum IgG antibody is detected by ELISA (enzyme-linked immunosorbent assay), spss software is used for analysis of data, and the results show that the rMUCIN protein and the rCp23 protein have the same positive rate in detection of unknown serum (XC2=0.370, and P=0.543>0.05), while the positive rate of the rMUCIN protein in detection of the unknown serum is higher than that of a cryptosporidium parvum crude antigen (XC2=5.222, and P=0.02<0.05). The invention further provides a kit for ELISA detection of the anti-cryptosporidium parvum IgG antibody, which takes the recombinant antigen rMUCIN as the detection antigen, takes the rCp23 as a positive control and has stronger specificity, sensitivity and reliability.
Owner:JILIN UNIV
Method for enriching Cryptosporidium parvum oocysts and Giardia Lamblia sporocysts in water
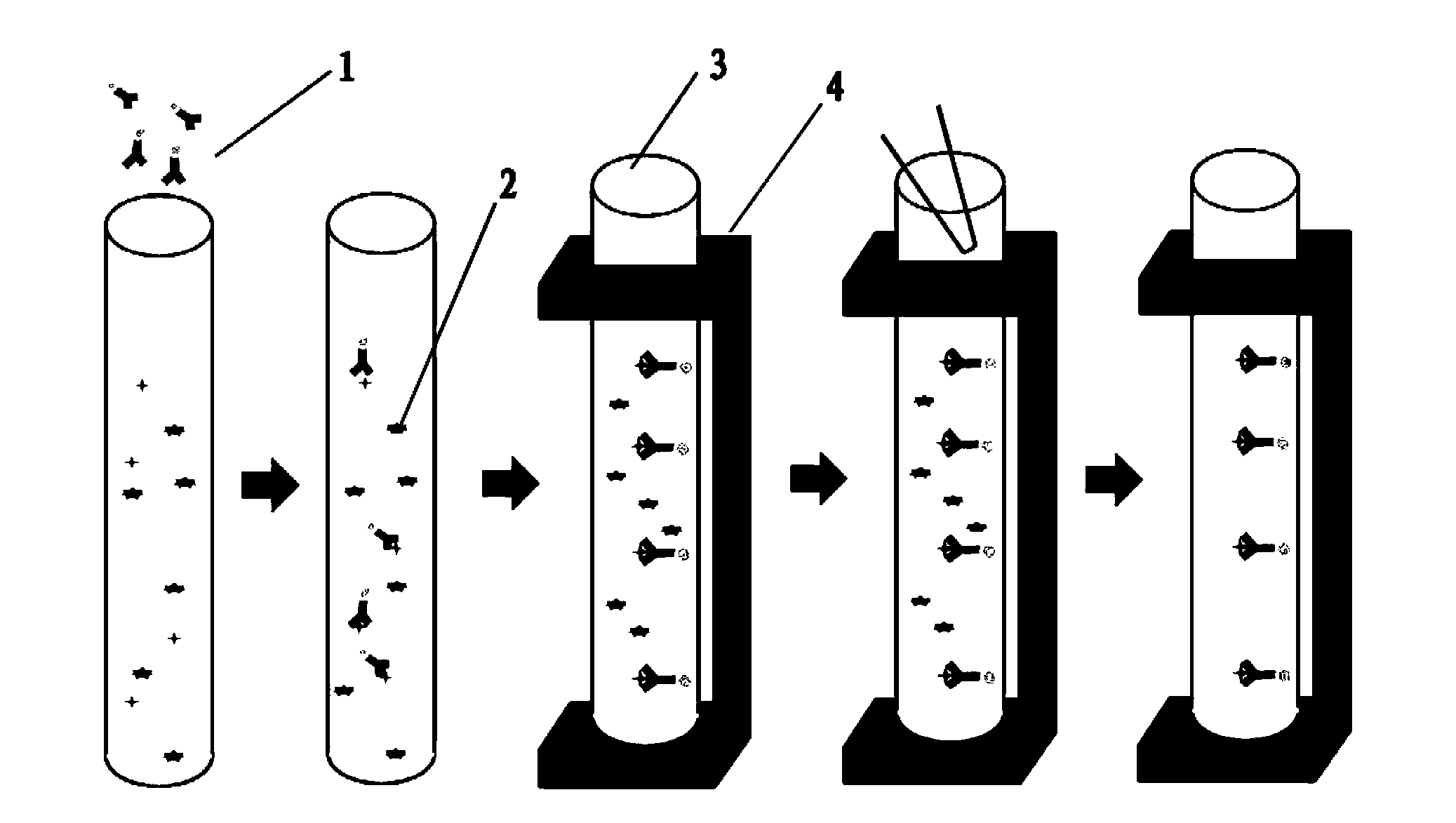
InactiveCN102191307AEfficient enrichmentReduce investmentMicrobiological testing/measurementPreparing sample for investigationCryptosporidium parvumImmunofluorescent labeling
The invention relates to a method for enriching Cryptosporidium parvum oocysts and Giardia Lamblia sporocysts in water. The method comprises the following steps: adding an inorganic metal salt coagulant into a bulk water sample, stirring, coagulating, precipitating, collecting precipitate alumen ustum in a small-volume container, adding acidic solution into the small-volume container to dissolve the alumen ustum, centrifuging, removing supernate and thus, enriching the Cryptosporidium parvum oocysts and Giardia Lamblia sporocysts in water. In the invention, the problems of low Cryptosporidium parvum oocyst and Giardia Lamblia sporocyst enrichment rate, instability, high operation requirement and expensive filtering equipment and consumable items of the conventional method for enriching Cryptosporidium parvum oocysts and Giardia Lamblia sporocysts. The method does not need expensive apparatus and equipment and medicines and greatly reduce capital investment. The method is simple in operation, easy to master, time-saving and labor-saving, reduces cost of enrichment of Cryptosporidium parvum oocysts and Giardia Lamblia sporocysts in water, improves enrichment efficiency and is a novel method which clears the preliminary work for subsequent steps of immunomagnetic separation, immunofluorescence label microscope counting, polymerase chain reaction (PCR) qualitative detection, nano probe gene chip detection and Real-time PCR quantitative detection and the like.
Owner:INST OF URBAN ENVIRONMENT CHINESE ACAD OF SCI
Compositions and vaccines containing antigen(s) of Cryptosporidium parvum and of another pathogen
InactiveUS20060286109A1Improved and useful productionHigh productSsRNA viruses positive-senseGenetic material ingredientsEpitopeMammal
Combination compositions including C. parvum antigen(s) or epitope(s) of interest with at least one other antigen or epitope of interest from a pathogen that causes enteric infection and / or symptoms and / or recombinant(s) and / or vector(s) and / or plasmid(s) expressing such antigen(s) or epitope(s) of interest and administration of such compositions such as to pregnant mammals and / or newborn or young mammals, for instance, pregnant cows and / or calves such as within the first month of birth, are disclosed and claimed.
Owner:AUDONNET JEAN CHRISTOPHE +1
LAMP primer composition for detecting two main parasites causing calf diarrhea and application of LAMP primer composition
ActiveCN107365843AMicrobiological testing/measurementAgainst vector-borne diseasesAgricultural scienceNucleotide
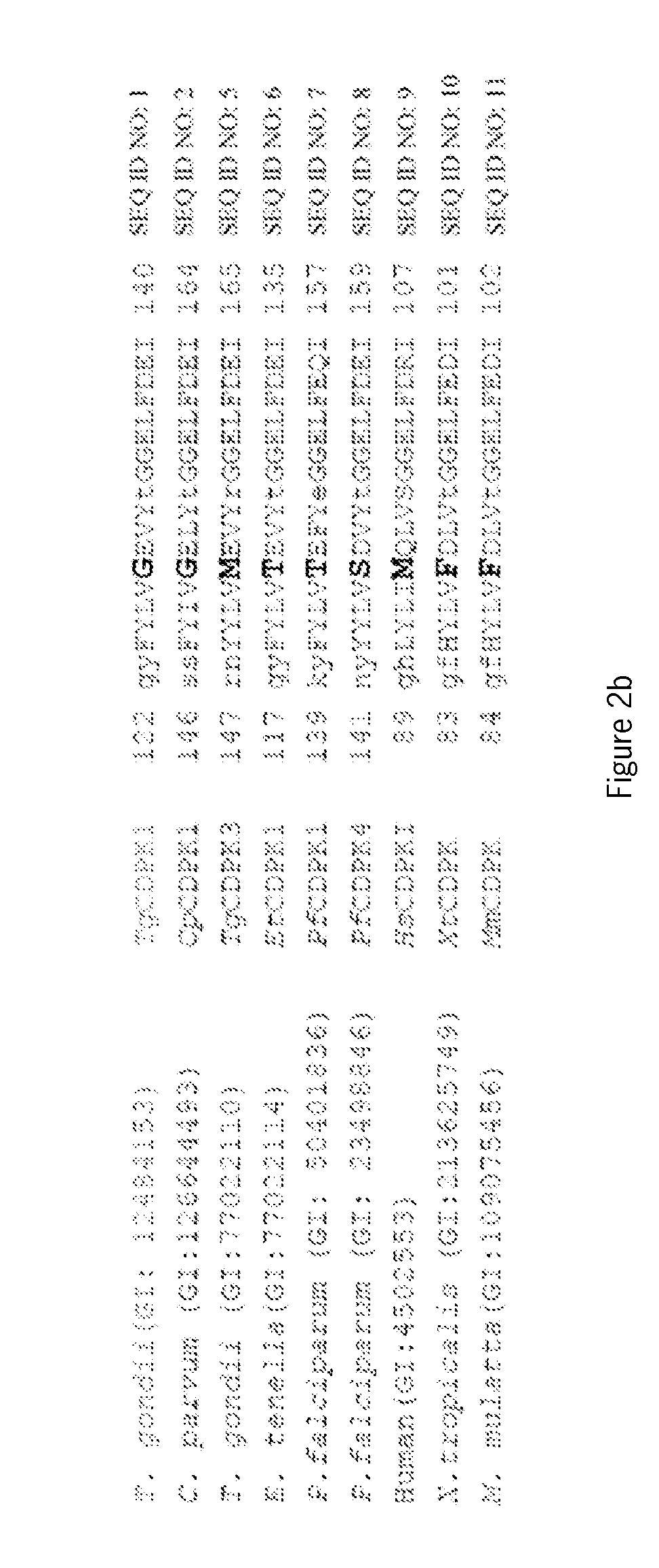
The invention relates to the technical field of biology and particularly discloses an LAMP primer composition for detecting two main parasites causing calf diarrhea and application of the LAMP primer composition. The LAMP primer composition for detecting the Eimeria bovis and / or Cryptosporidium parvum which cause the calf diarrhea is a primer group I and / or a primer group II. The primer group I comprises a primer I-F3, a primer I-B3, a primer I-FIP, a primer I-BIP, a primer I-LF and a primer I-LB, and the nucleotide sequences of the primers are as shown in SEQ ID No. 1-6. The primer group II comprises a primer II-F3, a primer II-B3, a primer II-FIP, a primer II-BIP, a primer II-LF and a primer II-LB, and the nucleotide sequences of the primers are as shown in SEQ ID No. 7-12. The LAMP primer composition is used for detecting two common parasites causing the calf diarrhea and is high in specificity and sensitivity, capable of achieving simple, fast and accurate detection and worthy of popularization.
Owner:HENAN ACAD OF AGRI SCI +2
Compositions and vaccines containing antigen(s) of cryptosporidium parvum and of another pathogen
InactiveUS20020086031A1Improved and useful productionHigh production of antibodySsRNA viruses positive-senseGenetic material ingredientsEpitopeMammal
Combination compositions including C. parvum antigen(s) or epitope(s) of interest with at least one other antigen or epitope of interest from a pathogen that causes enteric infection and / or symptoms and / or recombinant(s) and / or vector(s) and / or plasmid(s) expressing such antigen(s) or epitope(s) of interest and administration of such compositions such as to pregnant mammals and / or newborn or young mammals, for instance, pregnant cows and / or calves such as within the first month of birth, are disclosed and claimed.
Owner:MERIAL SAS
High resolution analysis of genetic variation within cryptosporidium parvum
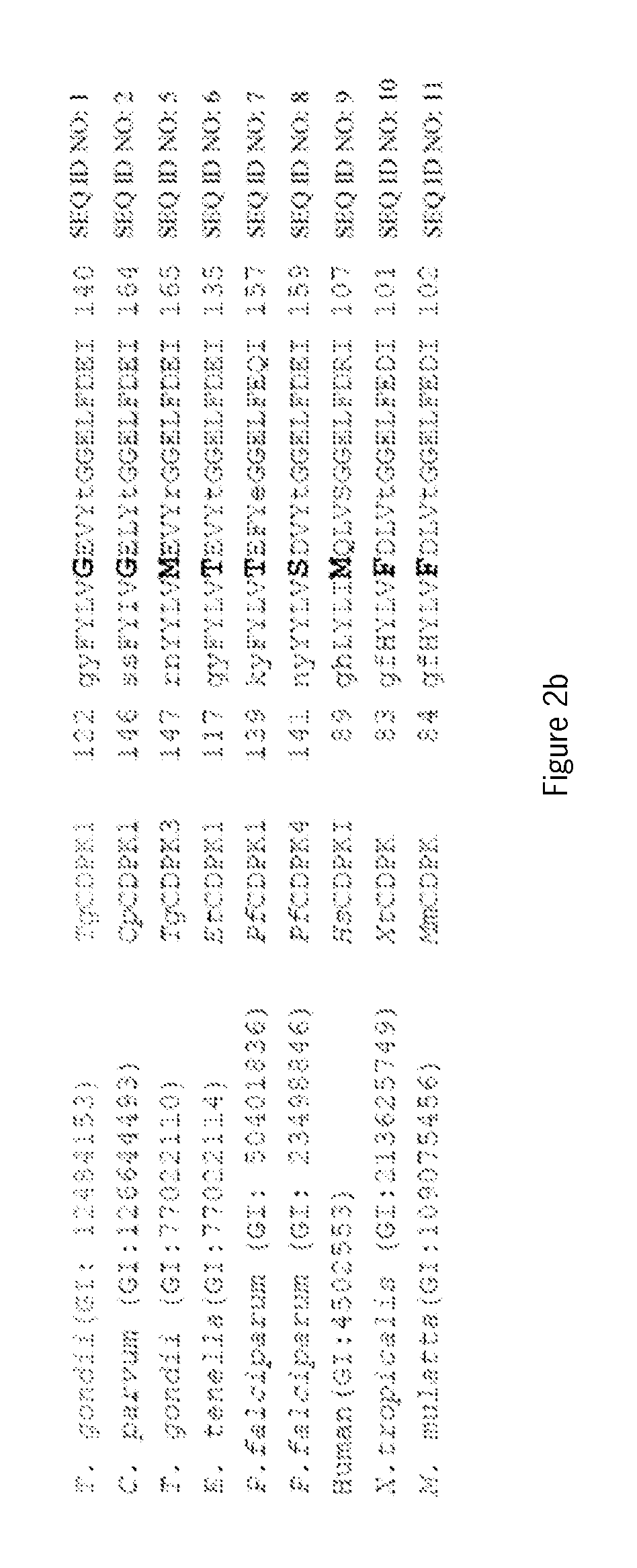
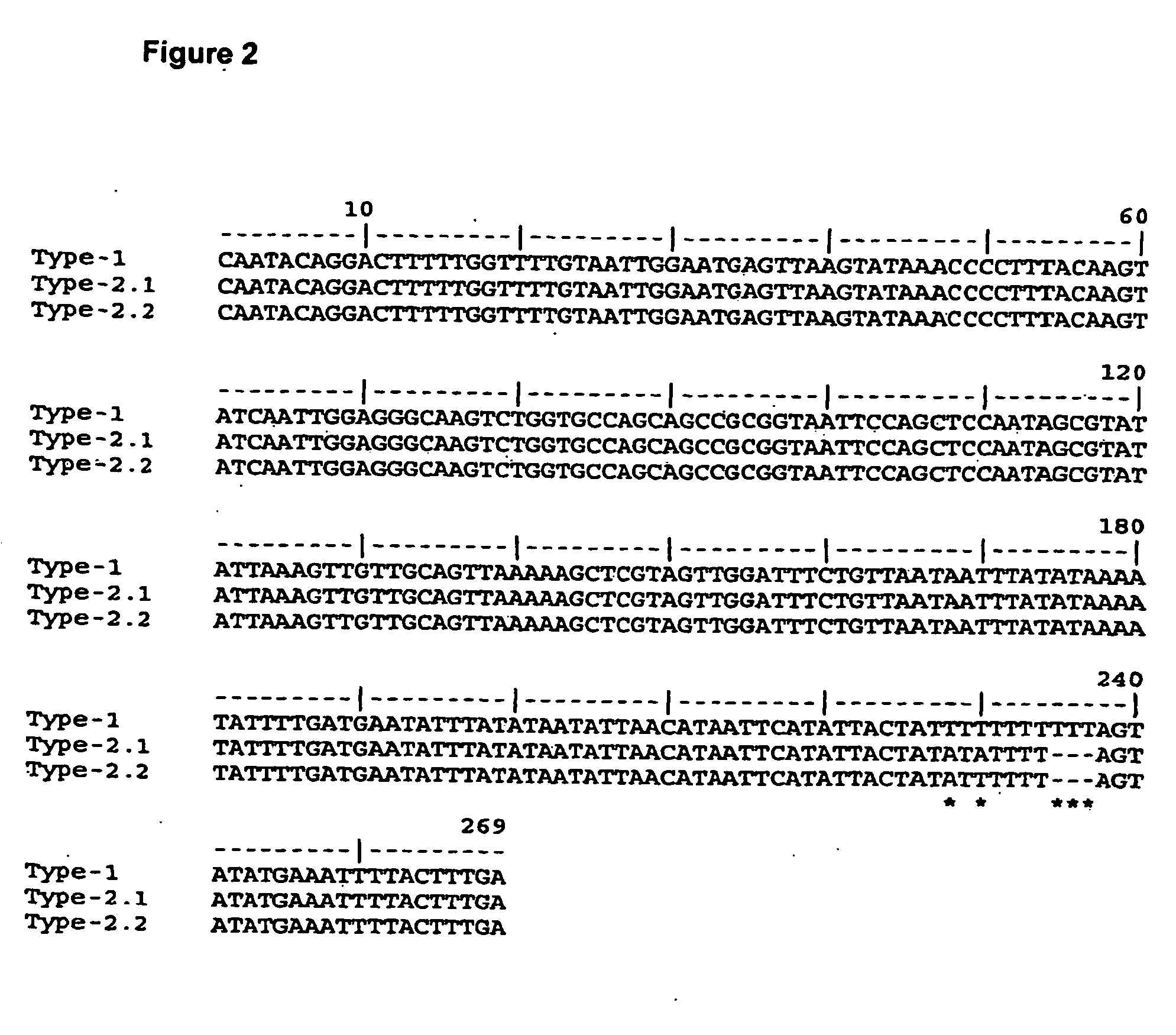
InactiveUS20060258855A1Sugar derivativesMicrobiological testing/measurementCryptosporidium parvumGenotype
An oligonucleotide selected from at least one of the DNA sequences designated SEQ ID NO: 3, SEQ ID NO: 4, SEQ ID NO: 5, and / or SEQ ID NO: 6, or annealing equivalents thereof is described. Also disclosed is a method for the genotypic and subgenotypic identification of the genus Cryptosporidium in a sample.
Owner:GENETYPE PTY LTD
CTL epitope peptide of cryptosporidium parvum and application and vaccine of CTL epitope peptide
ActiveCN109942693AHigh affinityAffinity, indicating that the corresponding immunological function is goodAntiparasitic agentsAntibody medical ingredientsCtl epitopeBiotechnology
The invention relates to a CTL epitope peptide of cryptosporidium parvum. The peptide has the amino acid sequence of SEQ ID NO.1. The invention further provides a nucleic acid for coding the CTL epitope peptide of cryptosporidium parvum. The nucleic acid has the nucleotide sequence of SEQ ID NO.2. The CTL epitope peptide P1 of cryptosporidium parvum has quite high affinity to H-2Kb molecules or HLA-A*0201 molecules, and it means that the CTL epitope peptide has the corresponding immunology functions. A new direction is provided for preparing a cryptosporidium parvum vaccine. The peptide presents excellent irritation reactions in the polypeptide specificity CTL immune reaction in C57BL / 6N mouse splenocytes and has huge development and application potential in the cryptosporidium parvum specific immunotherapy field.
Owner:ZHOUKOU NORMAL UNIV
Process and apparatus for removal of biocolloids from water
InactiveUS20070210009A1Eliminate needWater treatment parameter controlWater/sewage treatment by irradiationCryptosporidium parvumFilter media
Biocolloids, e.g. Cryptosporidium parvum oocysts, are removed from water by filtration using a packed bed of a granular filter medium, preferably MgO, establishing an electric field across the packed bed, perpendicular to the flow of the water through the packed bed. The packed bed is provided in an annular space between two concentric electrodes.
Owner:ENVIRONMENTAL PROTECTION AGENCY US
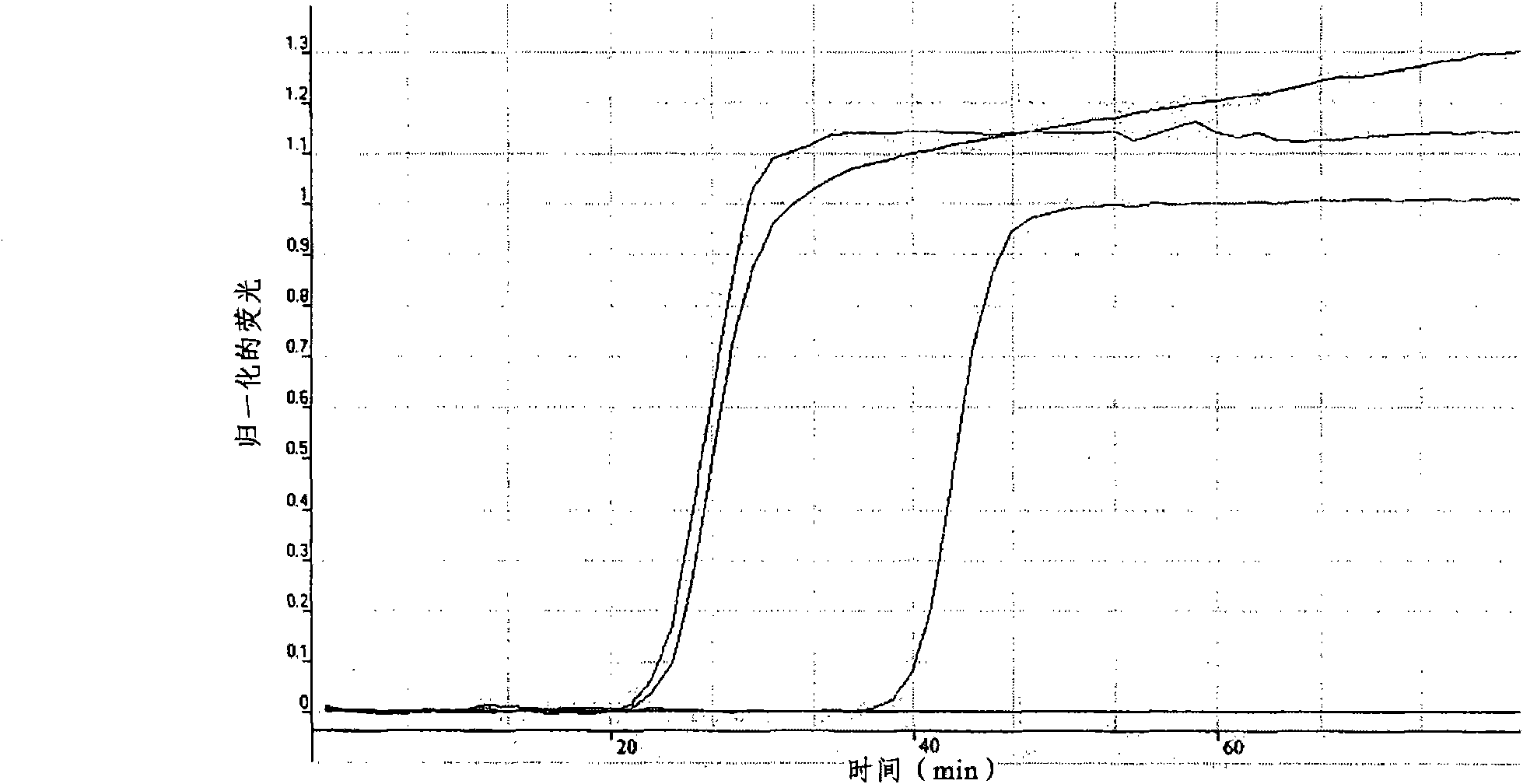
A fluorescence LAMP detection method for Cryptosporidium parvum
InactiveCN108893526AReduce testing costsQuick checkMicrobiological testing/measurementMicroorganism based processesHsp70 geneFluorescence
The invention provides a fluorescence LAMP detection method for Cryptosporidium parvum and the method is specific, sensitive, low in the detection cost, short in time consuming, high in efficiency, and simple in operation. An adopted technical scheme includes the following steps: (1): screening and determination of specific primers of genetic loci which are SSU RNA, COWP, actin and HSP70 of the Cryptosporidium parvum; (2): design and synthesis of fluorescence LAMP primers of Cryptosporidium parvum, optimization of a reaction condition and drafting of a standard curve and a melting curve; and (3): testing of specificity, sensitivity and repeatability and detection application for clinical samples.
Owner:NORTHWEST A & F UNIV
Compositions and methods for treating toxoplasmosis, cryptosporidiosis and other apicomplexan protozoan related diseases
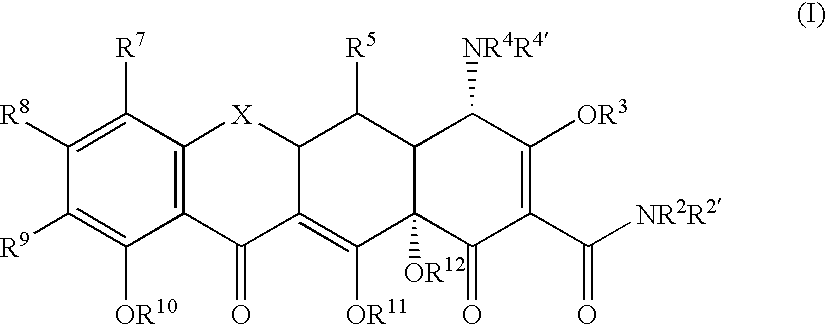
The present disclosure is directed to compositions and methods for inhibiting either Toxoplasma gondii (T. gondii) calcium dependent protein kinases (TgCDPKs) or Cryptosporidium parvum (C. parvum) and Cryptosporidium hominus (C. hominus) calcium dependent protein kinases (CpCDPKs) using pyrazolopyrimidine and / or imidazo[1,5-α]pyrazine inhibitors, of the Formula (I), wherein the variables X, Y, Z, R1, and R3 are defined herein.
Owner:UNIV OF WASHINGTON
Application of alantolactone in preparation of medicines for resisting cryptosporidium parvum
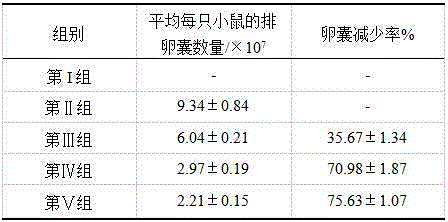
ActiveCN104666295ADecreased ovulation sac volumeGood against CryptosporidiumOrganic active ingredientsAnimal feeding stuffBiotechnologyCryptosporidium parvum
The invention discloses an application of alantolactone in preparation of medicines for resisting cryptosporidium parvum. The application method comprises the following steps: preparing powder from alantolactone and acceptable auxiliary materials; adding to a feed, mixing evenly, and feeding mouse according to the daily feed amount; or preparing an alantolactone water-soluble power from the alantolactone, dissolving into water, and feeding the mouse according to the daily water consumption. An animal infection experiment proves that the oocysts after cryptosporidium parvum injection can be reduced by 75% or above by the alantolactone; and the alantolactone has a relatively good effect of resisting cryptosporidium parvum.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
Process and apparatus for removal of biocolloids from water
InactiveUS7811460B2Eliminate needWater treatment parameter controlWater/sewage treatment by irradiationCryptosporidium parvumFilter media
Biocolloids, e.g. Cryptosporidium parvum oocysts, are removed from water by filtration using a packed bed of a granular filter medium, preferably MgO, establishing an electric field across the packed bed, perpendicular to the flow of the water through the packed bed. The packed bed is provided in an annular space between two concentric electrodes.
Owner:ENVIRONMENTAL PROTECTION AGENCY US
Multiplex RT-PCR/PCR for simultaneous detection of bovine coronavirus, bovine rotavirus, Cryptosporidium parvum, and Escherichia coli
InactiveUS20050239057A1Rapid and sensitive and specificRapid and sensitive and detectionMicrobiological testing/measurementFermentationEscherichia coliBovine rotavirus
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
Cryptosporidium and cryptosporidium parvum specific PCR detecting reagent kit and detecting method
InactiveCN101353690BRigorous designEasy to operateMicrobiological testing/measurementAgainst vector-borne diseasesPositive controlCryptosporidium infection
The invention provides a peculiar PCR detection kit of Cryptosporidium and micro Cryptosporidium, comprising a DNA lysis solution, a PCR reaction solution, a peculiar primer of the Cryptosporidium and the micro Cryptosporidium and positive control genomic DNA of the micro Cryptosporidium. According to the diagnosing sequence for analyzing Cryptosporidium and species peculiarities, a Cryptosporidium and species peculiar primer are designed by utilizing a peculiar detection method of PCR, the sensibility of the primer is improved by optimizing and amplifying conditions, the amplifying result can be directly observed by electrophoretic analysis. The kit of the invention has strict design, simple and easy operation, high sensibility and strong peculiarity, can simultaneously identify the Cryptosporidium infection of Cryptosporidium and species peculiarities so as to obtain an accurate and objective judgment result.
Owner:JILIN UNIV
Multiple PCR (polymerase chain reaction) detection kit and method for giardia and cryptosporidia of cattle
InactiveCN108796106AEasy to observe the resultsObservation results are quickMicrobiological testing/measurementAgainst vector-borne diseasesConserved sequenceCryptosporidium parvum
The invention discloses a multiple PCR (polymerase chain reaction) detection kit and method for giardia and cryptosporidia of cattle. According to parts of conserved sequences of giardia duodenalis TPI genes, cryptosporidium parvum 18S rRNA genes and cryptosporidium andersoni 18S rRNA genes, a primer is designed, a triple PCR detection method is built, sensitivity and accuracy of the method are improved by optimizing reaction conditions such as annealing temperature of PCR reaction, amplification products are implemented by 1.0% of agarose gel electrophoresis, and a result is conveniently andrapidly observed. Three target fragments amplified by the method have size differences in agarose gel electrophoresis and are easily distinguished, the sensitivity can reach 1*10<3> / g and is higher than that of a reported microscopy method and a conventional PCR detection method of giardia duodenalis, cryptosporidium parvum and cryptosporidium andersoni, and the method is suitable for detection ofsamples in clinical practice. The detection method can simultaneously and rapidly detect the giardia duodenalis, the cryptosporidium parvum and the cryptosporidium andersoni of the cattle and has theadvantages of simplicity in operation, high sensitivity, high specificity and the like, and technical support is provided for quality standards of real experiment cattle.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com