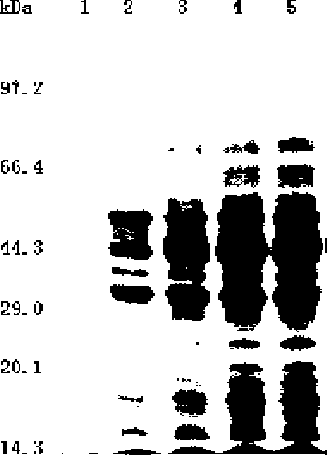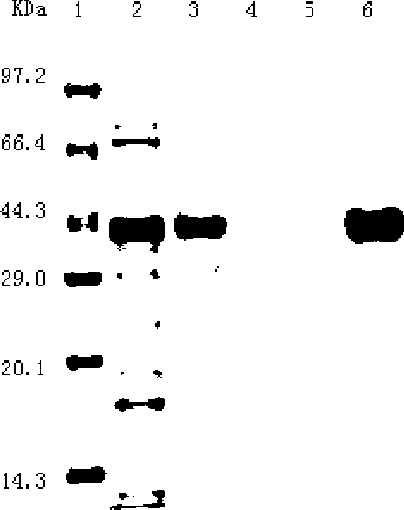Echinococcosis antigen gene (egG1Y162 antigen gene), and recombinant protein and use thereof
An egg1y162, antigen gene technology, applied in application, gene therapy, genetic engineering and other directions, can solve problems such as not achieving ideal results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
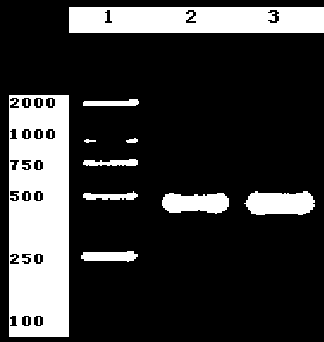
[0019] Embodiment 1: utilize polymerase chain reaction (PCR) technology to amplify egG1Y162 antigen gene of the present invention
[0020] First RNA (ribonucleic acid) extraction and PCR amplification of the egG1Y162 gene of the present invention: get 100 mg of protocercaria (derived from the liver of sheep infected with Echinococcus granulosus), add 1 milliliter of Trizol (can destroy the cell wall to release the ribonucleic acid) A kind of lysate that came out) was fully homogenized, and stood at room temperature for 5 minutes; added 0.2 ml of chloroform, oscillated for 15 seconds, and stood for 2 minutes; centrifuged at 4 degrees, 12000 g × 15 minutes, took the supernatant; Alcohol, mix the liquid in the tube gently, let it stand at room temperature for 10 minutes; centrifuge at 4 degrees, 12000 g × 10 minutes, discard the supernatant; add 1 ml of 75% ethanol, gently wash the precipitate, 4 degrees, 7500 g × 5 Minutes, discard the supernatant, dry it, and add 30 microliters...
Embodiment 2
[0024] Example 2: Obtaining a large number of egG1Y162 gene sequences of the present invention
[0025] The egG1Y162 gene of the present invention and the PUCm-T vector (a general cloning vector) were ligated overnight at 16°C under the action of T4 DNA ligase. The egG1Y162 target gene of the present invention is constructed on the PUCm-T vector, the PUCm-T / egY162 recombinant plasmid is constructed, and the recombinant plasmid is transformed into E. Coli DH5α (for cloning E. coli), and then plated and cultured overnight at 37 degrees , so that a large number of recombinant plasmids can be replicated in bacteria. 5 colonies were randomly selected, plasmids were extracted, and gene sequencing was carried out. The results were compared with online gene databases and found that the egG1Y162 gene of the present invention is a new gene found in Echinococcus granulosus.
Embodiment 3
[0026] Embodiment 3: obtain egG1Y162 recombinant protein of the present invention
[0027] It is necessary to construct the PET-41a / egG1Y162 prokaryotic expression plasmid, use EcoR I / Hind III to cut the PUCm-T / egG1Y162 recombinant plasmid and the prokaryotic expression plasmid pET41a, recover, connect, and transform into E.coli BL21 (a gene with expression function) coli) at 37°C overnight. The recombinant plasmid was amplified by PCR, identified by EcoRI / Hind III double enzyme digestion, and then sequenced. Sequencing correct PET-41a / egG1Y162 prokaryotic expression plasmid, 30 degrees, induced by IPTG (final concentration of 0.5 mmol / L isopropyl-β-D-thiogalactoside, protein inducer) for 0 hours, 2 Hours, 4 hours, 6 hours, collect the bacteria, centrifuge at 4 degrees, 12000 rpm for 10 minutes, and use 12% SDS-PAGE (polypropylene gel electrophoresis analysis) to detect, the mobility of proteins with different molecular weights is different, Then stain with Coomassie Brillia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com